A-431
Target ID: CHEMBL614069
Organism: Homo sapiens
Type: CELL-LINE
Molecules
| Name | SMILES | Bioactivity | Structure |
|---|---|---|---|
| ERLOTINIB | C#Cc1cccc(Nc2ncnc3cc(OCCOC)c(OCCOC)cc23)c1 | IC50: 1450.0 nM | 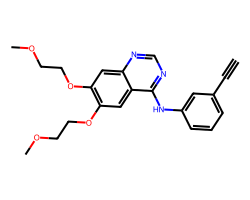 |
| CANERTINIB DIHYDROCHLORIDE | C=CC(=O)Nc1cc2c(Nc3ccc(F)c(Cl)c3)ncnc2cc1OCCCN1CCOCC1.Cl.Cl | IC50: 1.5 nM | 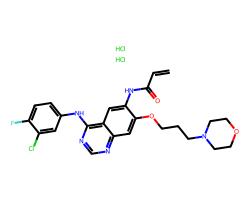 |
| ALVOCIDIB | CN1CC[C@H](c2c(O)cc(O)c3c(=O)cc(-c4ccccc4Cl)oc23)[C@H](O)C1 | IC50: 330.0 nM | 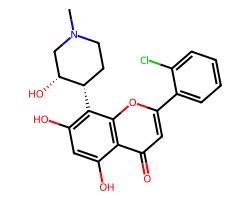 |
| CANERTINIB | C=CC(=O)Nc1cc2c(Nc3ccc(F)c(Cl)c3)ncnc2cc1OCCCN1CCOCC1 | IC50: 74.0 nM | 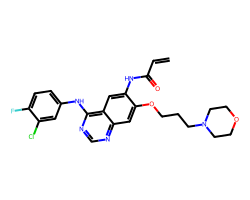 |
| GEFITINIB | COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1OCCCN1CCOCC1 | IC50: 515.0 nM | 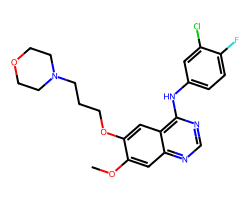 |
| TAMOXIFEN | CC/C(=C(\c1ccccc1)c1ccc(OCCN(C)C)cc1)c1ccccc1 | IC50: 6750.0 nM | 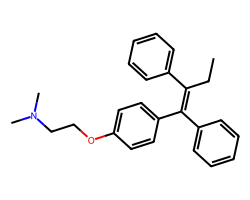 |
| PELITINIB | CCOc1cc2ncc(C#N)c(Nc3ccc(F)c(Cl)c3)c2cc1NC(=O)/C=C/CN(C)C | IC50: 83.0 nM | 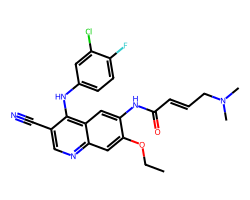 |
Top Fragments
| SMILES | Avg pKi | Count | Structure |
|---|---|---|---|
| [3*]O[3*] | 6.8 | 223 | 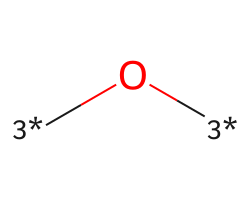 |
| [5*]N[5*] | 7.03 | 195 | 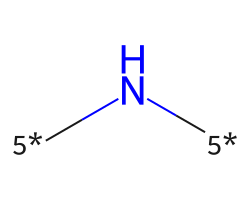 |
| [14*]c1ncnc2cc([16*])c([16*])cc12 | 7.02 | 156 | 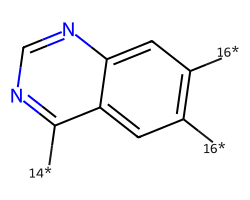 |
| [16*]c1ccc(F)c(Cl)c1 | 7.33 | 156 | 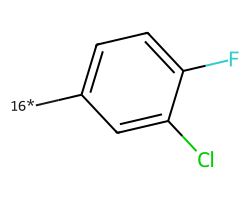 |
| [5*]N1CCOCC1 | 7.41 | 117 | 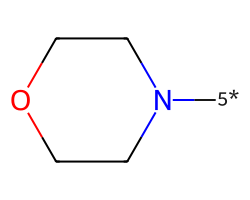 |