Hepatocyte
Target ID: CHEMBL613690
Organism: Homo sapiens
Type: CELL-LINE
Molecules
| Name | SMILES | Bioactivity | Structure |
|---|---|---|---|
| FUROSEMIDE | NS(=O)(=O)c1cc(C(=O)O)c(NCc2ccco2)cc1Cl | Ki: 100000.0 nM | 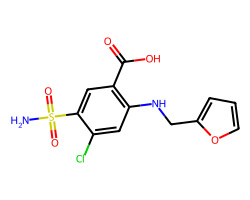 |
| VORINOSTAT | O=C(CCCCCCC(=O)Nc1ccccc1)NO | Inhibition: 100.0 % | 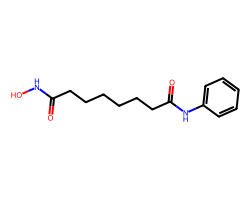 |
| ROSUVASTATIN | CC(C)c1nc(N(C)S(C)(=O)=O)nc(-c2ccc(F)cc2)c1/C=C/[C@@H](O)C[C@@H](O)CC(=O)O | Ki: 0.9 nM | 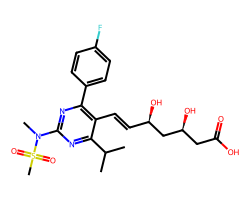 |
| THEOPHYLLINE | Cn1c(=O)c2[nH]cnc2n(C)c1=O | ED50: 18.5 mg.kg-1 | 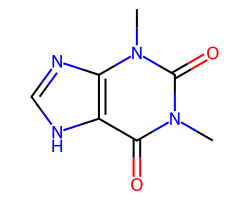 |
| ACETAMINOPHEN | CC(=O)Nc1ccc(O)cc1 | Vdss: 0.95 L.kg-1 | 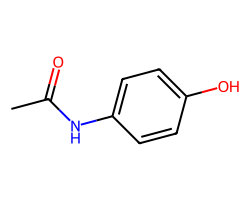 |
| CAFFEINE | Cn1c(=O)c2c(ncn2C)n(C)c1=O | Vdss: 0.61 L.kg-1 | 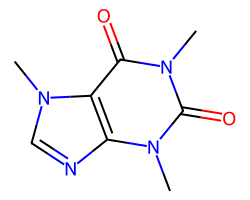 |
| SILDENAFIL | CCCc1nn(C)c2c(=O)[nH]c(-c3cc(S(=O)(=O)N4CCN(C)CC4)ccc3OCC)nc12 | Pc: 87000000.0 cm s-1 | 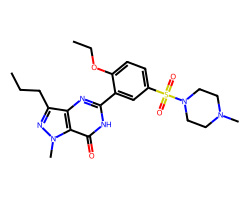 |
| LINIFANIB | Cc1ccc(F)c(NC(=O)Nc2ccc(-c3cccc4[nH]nc(N)c34)cc2)c1 | IC50: 4.0 nM | 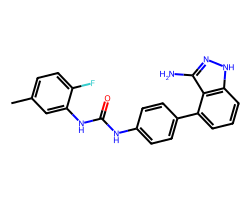 |
| TOFACITINIB | C[C@@H]1CCN(C(=O)CC#N)C[C@@H]1N(C)c1ncnc2[nH]ccc12 | IC50: 13.0 nM | 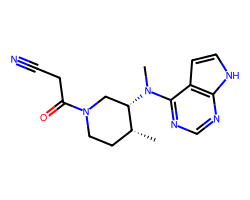 |
Top Fragments
| SMILES | Avg pKi | Count | Structure |
|---|---|---|---|
| [5*]N[5*] | 6.72 | 114 | 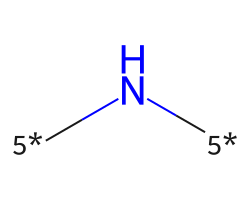 |
| [5*]N([5*])C | 8.27 | 58 | 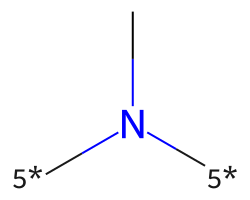 |
| c1ccccc1 | None | 51 | 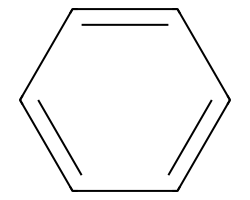 |
| O=c1[nH]c(=O)c2[nH]cnc2[nH]1 | None | 44 | 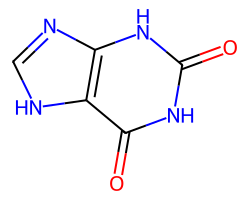 |
| [16*]c1ccccc1 | None | 39 | 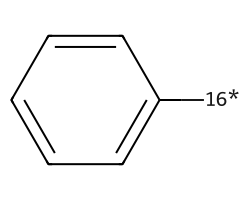 |