Bile salt export pump
Target ID: CHEMBL6020
Organism: Homo sapiens
Type: SINGLE PROTEIN
Molecules
| Name | SMILES | Bioactivity | Structure |
|---|---|---|---|
| METHOTREXATE | CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(C(=O)N[C@@H](CCC(=O)O)C(=O)O)cc1 | ID50: 6.2 nM | 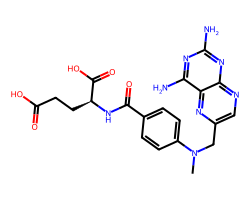 |
| FUROSEMIDE | NS(=O)(=O)c1cc(C(=O)O)c(NCc2ccco2)cc1Cl | Ki: 100000.0 nM | 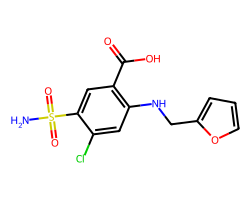 |
| PAPAVERINE | COc1ccc(Cc2nccc3cc(OC)c(OC)cc23)cc1OC | Blood pressure: -23.0 mmHg | 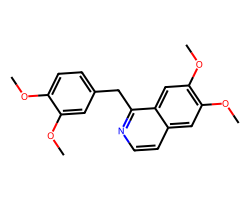 |
| FLUVASTATIN | CC(C)n1c(/C=C/C(O)CC(O)CC(=O)O)c(-c2ccc(F)cc2)c2ccccc21 | IC50: 28.0 nM | 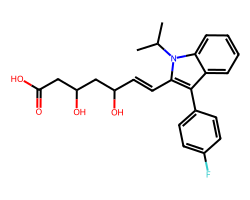 |
| INAMRINONE | Nc1cc(-c2ccncc2)c[nH]c1=O | Mean ED50: 0.389 mg kg-1 | 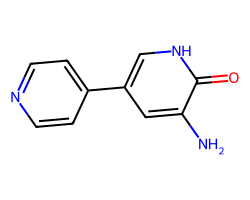 |
| GUAIFENESIN | COc1ccccc1OCC(O)CO | Inhibition: 5.0 % | 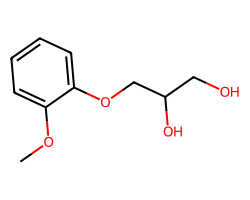 |
| ERLOTINIB | C#Cc1cccc(Nc2ncnc3cc(OCCOC)c(OCCOC)cc23)c1 | IC50: 1450.0 nM | 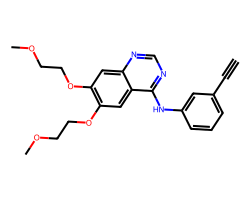 |
| HISTAMINE | NCCc1c[nH]cn1 | KA: 2.0 uM | 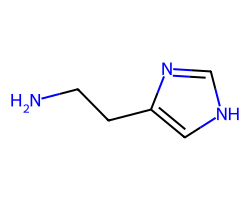 |
| AMPRENAVIR | CC(C)CN(C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)O[C@H]1CCOC1)S(=O)(=O)c1ccc(N)cc1 | Ki: 0.16 nM |  |
| AMITRIPTYLINE | CN(C)CCC=C1c2ccccc2CCc2ccccc21 | IC50: 61.0 nM |  |
| ACETAZOLAMIDE | CC(=O)Nc1nnc(S(N)(=O)=O)s1 | Ki: 250.0 nM |  |
| CARBIMAZOLE | CCOC(=O)n1ccn(C)c1=S | IC50: 10400.0 nM |  |
| EMETINE | CC[C@H]1CN2CCc3cc(OC)c(OC)cc3[C@@H]2C[C@@H]1C[C@H]1NCCc2cc(OC)c(OC)cc21 | IC50: 40.0 nM | 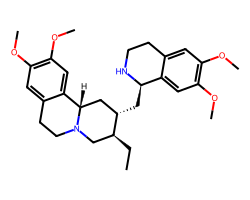 |
| THEOPHYLLINE | Cn1c(=O)c2[nH]cnc2n(C)c1=O | ED50: 18.5 mg.kg-1 | 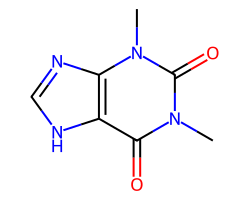 |
| AMIKACIN | NCC[C@H](O)C(=O)N[C@@H]1C[C@H](N)[C@@H](O[C@H]2O[C@H](CN)[C@@H](O)[C@H](O)[C@H]2O)[C@H](O)[C@H]1O[C@H]1O[C@H](CO)[C@@H](O)[C@H](N)[C@H]1O | C50: 0.21 uM | 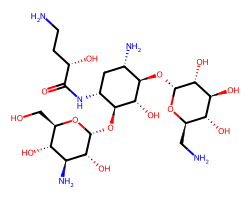 |
| TOPIRAMATE | CC1(C)O[C@@H]2[C@@H](CO[C@@]3(COS(N)(=O)=O)OC(C)(C)O[C@@H]23)O1 | Ki: 250.0 nM | 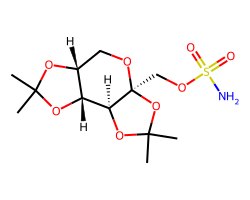 |
| ACETAMINOPHEN | CC(=O)Nc1ccc(O)cc1 | Vdss: 0.95 L.kg-1 |  |
| DICHLORPHENAMIDE | NS(=O)(=O)c1cc(Cl)c(Cl)c(S(N)(=O)=O)c1 | Ki: 1200.0 nM |  |
| FLUNARIZINE | Fc1ccc(C(c2ccc(F)cc2)N2CCN(C/C=C/c3ccccc3)CC2)cc1 | IC50: 290.0 nM |  |
| ETHYNODIOL DIACETATE | C#C[C@]1(OC(C)=O)CC[C@H]2[C@@H]3CCC4=C[C@@H](OC(C)=O)CC[C@@H]4[C@H]3CC[C@@]21C | Potency: 15848.9 nM |  |
| AMOXICILLIN | CC1(C)S[C@@H]2[C@H](NC(=O)[C@H](N)c3ccc(O)cc3)C(=O)N2[C@H]1C(=O)O | MIC: 0.021 ug.mL-1 | 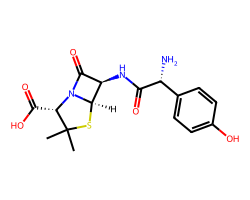 |
| ASTEMIZOLE | COc1ccc(CCN2CCC(Nc3nc4ccccc4n3Cc3ccc(F)cc3)CC2)cc1 | Binding energy: 11.3 kCal mol-1 | 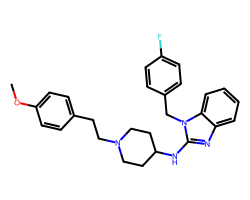 |
| ATROPINE | CN1[C@@H]2CC[C@H]1C[C@@H](OC(=O)C(CO)c1ccccc1)C2 | Ki: 0.45 nM | 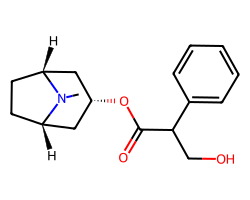 |
| CAFFEINE | Cn1c(=O)c2c(ncn2C)n(C)c1=O | Vdss: 0.61 L.kg-1 | 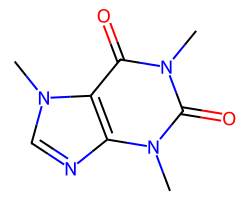 |
| QUERCETIN | O=c1c(O)c(-c2ccc(O)c(O)c2)oc2cc(O)cc(O)c12 | IC50: 32870.0 nM | 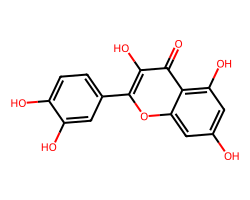 |
| AMILORIDE | N=C(N)NC(=O)c1nc(Cl)c(N)nc1N | Pc: 780000.0 cm s-1 | 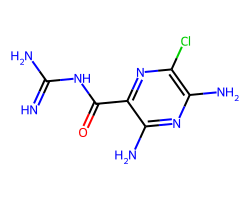 |
| LAPATINIB | CS(=O)(=O)CCNCc1ccc(-c2ccc3ncnc(Nc4ccc(OCc5cccc(F)c5)c(Cl)c4)c3c2)o1 | IC50: 10.0 nM | 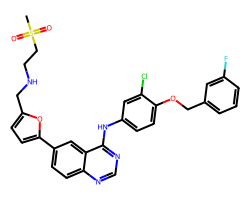 |
| TRIMETHOPRIM | COc1cc(Cc2cnc(N)nc2N)cc(OC)c1OC | IC50: 12000.0 nM | 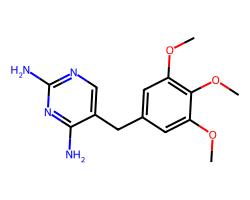 |
| NAFTOPIDIL | COc1ccccc1N1CCN(CC(O)COc2cccc3ccccc23)CC1 | Ki: 39.0 nM |  |
| IMATINIB | Cc1ccc(NC(=O)c2ccc(CN3CCN(C)CC3)cc2)cc1Nc1nccc(-c2cccnc2)n1 | IC50: 40.0 nM |  |
| GEFITINIB | COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1OCCCN1CCOCC1 | IC50: 515.0 nM |  |
| TAMOXIFEN | CC/C(=C(\c1ccccc1)c1ccc(OCCN(C)C)cc1)c1ccccc1 | IC50: 6750.0 nM |  |
| SORAFENIB | CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(C(F)(F)F)c3)cc2)ccn1 | IC50: 12.0 nM | 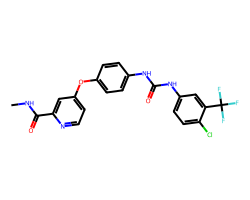 |
| ESTRONE | C[C@]12CC[C@@H]3c4ccc(O)cc4CC[C@H]3[C@@H]1CCC2=O | EC50: 1135.0 nM | 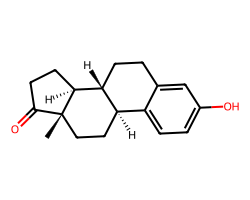 |
| PINACIDIL ANHYDROUS | CC(N/C(=N\C#N)Nc1ccncc1)C(C)(C)C | IC50: 800.0 nM | 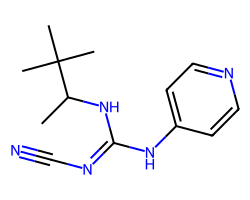 |
| MIBEFRADIL | COCC(=O)O[C@]1(CCN(C)CCCc2nc3ccccc3[nH]2)CCc2cc(F)ccc2[C@@H]1C(C)C | Inhibition: 86.0 % | 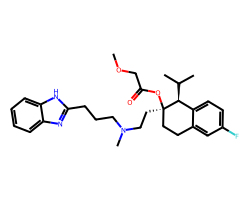 |
Top Fragments
| SMILES | Avg pKi | Count | Structure |
|---|---|---|---|
| [5*]N[5*] | 7.1 | 350 | 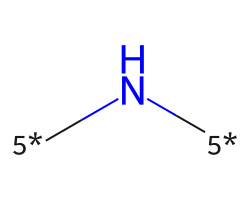 |
| [3*]O[3*] | 6.93 | 344 | 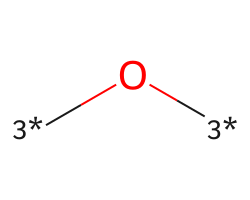 |
| [3*]OC | 6.23 | 277 | 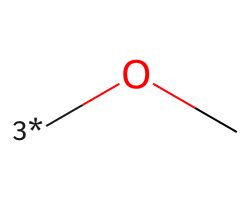 |
| [4*]C[8*] | 7.09 | 176 | 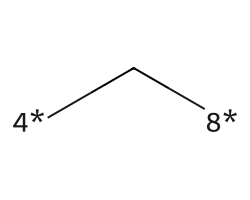 |
| [1*]C([6*])=O | 7.84 | 155 |  |