Serine/threonine-protein kinase Sgk2
Target ID: CHEMBL5794
Organism: Homo sapiens
Type: SINGLE PROTEIN
Molecules
| Name | SMILES | Bioactivity | Structure |
|---|---|---|---|
| TANDUTINIB | COc1cc2c(N3CCN(C(=O)Nc4ccc(OC(C)C)cc4)CC3)ncnc2cc1OCCCN1CCCCC1 | IC50: 26.0 nM | 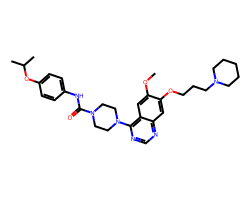 |
| ERLOTINIB | C#Cc1cccc(Nc2ncnc3cc(OCCOC)c(OCCOC)cc23)c1 | IC50: 1450.0 nM | 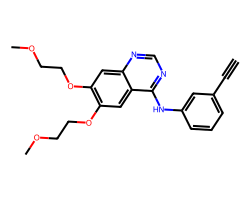 |
| AST-487 | CCN1CCN(Cc2ccc(NC(=O)Nc3ccc(Oc4cc(NC)ncn4)cc3)cc2C(F)(F)F)CC1 | Kd: 2.3 nM | 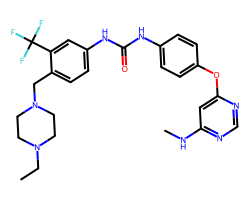 |
| LINIFANIB | Cc1ccc(F)c(NC(=O)Nc2ccc(-c3cccc4[nH]nc(N)c34)cc2)c1 | IC50: 4.0 nM | 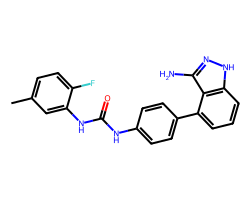 |
| VATALANIB | Clc1ccc(Nc2nnc(Cc3ccncc3)c3ccccc23)cc1 | IC50: 37.0 nM |  |
| IMATINIB | Cc1ccc(NC(=O)c2ccc(CN3CCN(C)CC3)cc2)cc1Nc1nccc(-c2cccnc2)n1 | IC50: 40.0 nM |  |
| MLN-8054 | O=C(O)c1ccc(Nc2ncc3c(n2)-c2ccc(Cl)cc2C(c2c(F)cccc2F)=NC3)cc1 | Activity: 39.7 % |  |
| SORAFENIB | CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(C(F)(F)F)c3)cc2)ccn1 | IC50: 12.0 nM |  |
Top Fragments
| SMILES | Avg pKi | Count | Structure |
|---|---|---|---|
| [5*]N[5*] | 7.6 | 312 | 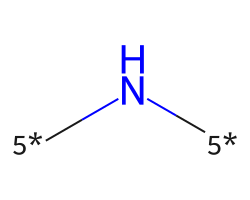 |
| [16*]c1ccc([16*])cc1 | 7.99 | 234 | 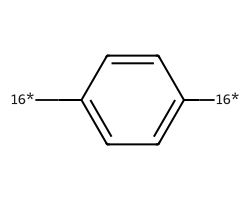 |
| [3*]O[3*] | 7.5 | 156 | 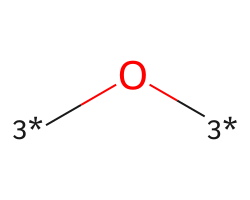 |
| [1*]C([1*])=O | 8.14 | 156 | 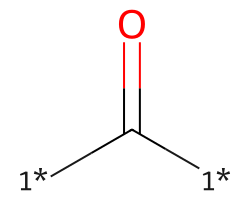 |
| [14*]c1ncnc2cc([16*])c([16*])cc12 | 6.71 | 78 |  |