DNA-(apurinic or apyrimidinic site) lyase
Target ID: CHEMBL5619
Organism: Homo sapiens
Type: SINGLE PROTEIN
Molecules
| Name | SMILES | Bioactivity | Structure |
|---|---|---|---|
| METHOTREXATE | CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(C(=O)N[C@@H](CCC(=O)O)C(=O)O)cc1 | ID50: 6.2 nM | 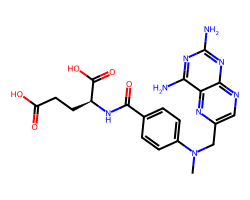 |
| BAICALEIN | O=c1cc(-c2ccccc2)oc2cc(O)c(O)c(O)c12 | Inhibition: 57.0 % | 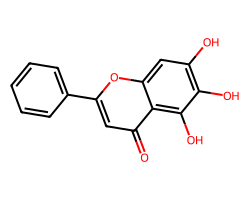 |
| CAFFEIC ACID | O=C(O)/C=C/c1ccc(O)c(O)c1 | IC50: 1200000.0 nM | 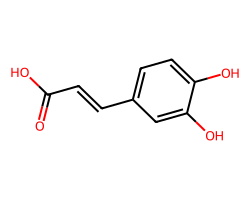 |
| QUERCETIN | O=c1c(O)c(-c2ccc(O)c(O)c2)oc2cc(O)cc(O)c12 | IC50: 32870.0 nM | 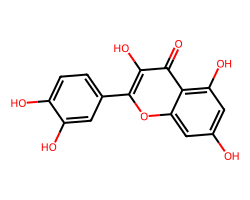 |
| HYDROCHLOROTHIAZIDE | NS(=O)(=O)c1cc2c(cc1Cl)NCNS2(=O)=O | IC50: 181970085860998.25 nM | 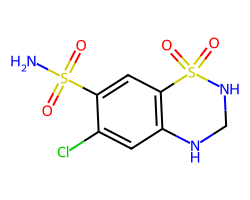 |
| AMINOPTERIN | Nc1nc(N)c2nc(CNc3ccc(C(=O)N[C@@H](CCC(=O)O)C(=O)O)cc3)cnc2n1 | Ki: 0.0037 nM | 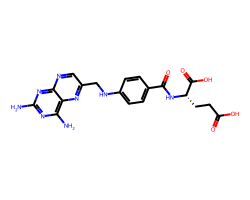 |
| DIPYRIDAMOLE | OCCN(CCO)c1nc(N2CCCCC2)c2nc(N(CCO)CCO)nc(N3CCCCC3)c2n1 | IC50: 500.0 nM | 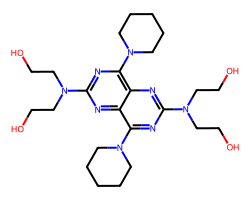 |
Top Fragments
| SMILES | Avg pKi | Count | Structure |
|---|---|---|---|
| c1ccc(NCc2cnc3ncncc3n2)cc1 | 9.82 | 78 | 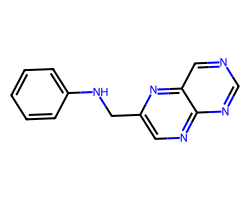 |
| [5*]N[5*] | 9.82 | 78 | 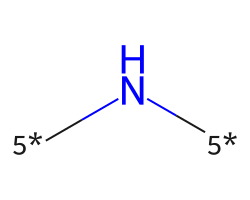 |
| [4*]C[8*] | 9.82 | 78 | 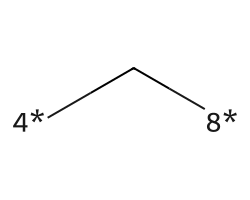 |
| [16*]c1ccc([16*])cc1 | 9.82 | 78 | 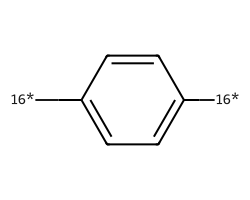 |
| [4*][C@@H](CCC(=O)O)C(=O)O | 9.82 | 78 | 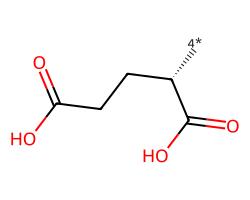 |