Gamma-aminobutyric acid receptor subunit alpha-1/alpha-2/beta-2/gamma-2
Target ID: CHEMBL5303741
Organism: Homo sapiens
Type: PROTEIN COMPLEX
Molecules
| Name | SMILES | Bioactivity | Structure |
|---|---|---|---|
| FLUOCINOLONE ACETONIDE | CC1(C)O[C@@H]2C[C@H]3[C@@H]4C[C@H](F)C5=CC(=O)C=C[C@]5(C)[C@@]4(F)[C@@H](O)C[C@]3(C)[C@]2(C(=O)CO)O1 | Potency: 35481.3 nM | 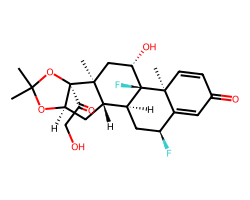 |
| FUROSEMIDE | NS(=O)(=O)c1cc(C(=O)O)c(NCc2ccco2)cc1Cl | Ki: 100000.0 nM | 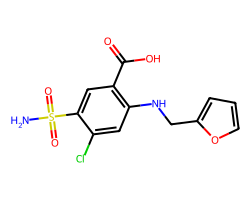 |
| FLUVASTATIN | CC(C)n1c(/C=C/C(O)CC(O)CC(=O)O)c(-c2ccc(F)cc2)c2ccccc21 | IC50: 28.0 nM | 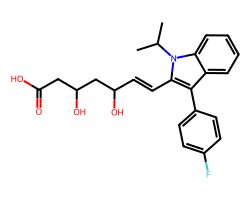 |
| HEXACHLOROPHENE | Oc1c(Cl)cc(Cl)c(Cl)c1Cc1c(O)c(Cl)cc(Cl)c1Cl | Control current: 237.4 % | 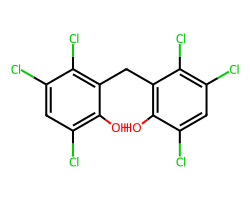 |
| GENTIAN VIOLET | CN(C)c1ccc(C(=C2C=CC(=[N+](C)C)C=C2)c2ccc(N(C)C)cc2)cc1.[Cl-] | Lysis: 100.0 % |  |
| GUAIFENESIN | COc1ccccc1OCC(O)CO | Inhibition: 5.0 % |  |
| EDARAVONE | CC1=NN(c2ccccc2)C(=O)C1 | PC50: 70.4 uM |  |
| MIFEPRISTONE | CC#C[C@]1(O)CC[C@H]2[C@@H]3CCC4=CC(=O)CCC4=C3[C@@H](c3ccc(N(C)C)cc3)C[C@@]21C | IC50: 0.028 nM |  |
| ERLOTINIB | C#Cc1cccc(Nc2ncnc3cc(OCCOC)c(OCCOC)cc23)c1 | IC50: 1450.0 nM | 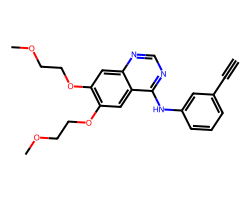 |
| VANDETANIB | COc1cc2/c(=N/c3ccc(Br)cc3F)nc[nH]c2cc1OCC1CCN(C)CC1 | IC50: 900.0 nM | 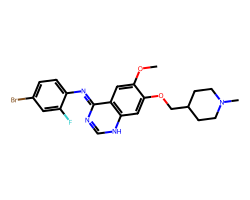 |
| VORINOSTAT | O=C(CCCCCCC(=O)Nc1ccccc1)NO | Inhibition: 100.0 % | 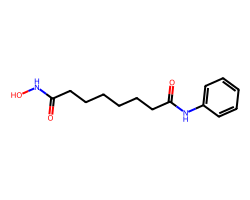 |
| DORZOLAMIDE | CCN[C@H]1C[C@H](C)S(=O)(=O)c2sc(S(N)(=O)=O)cc21 | Ki: 50000.0 nM | 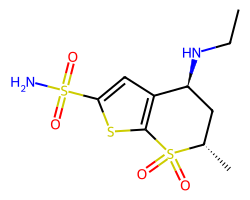 |
| HISTAMINE | NCCc1c[nH]cn1 | KA: 2.0 uM | 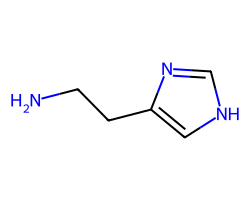 |
| AMITRIPTYLINE | CN(C)CCC=C1c2ccccc2CCc2ccccc21 | IC50: 61.0 nM | 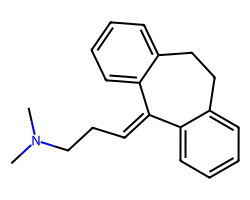 |
| ROSUVASTATIN | CC(C)c1nc(N(C)S(C)(=O)=O)nc(-c2ccc(F)cc2)c1/C=C/[C@@H](O)C[C@@H](O)CC(=O)O | Ki: 0.9 nM | 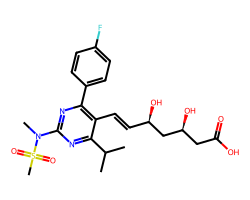 |
| EMETINE | CC[C@H]1CN2CCc3cc(OC)c(OC)cc3[C@@H]2C[C@@H]1C[C@H]1NCCc2cc(OC)c(OC)cc21 | IC50: 40.0 nM | 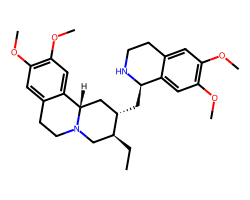 |
| BRIMONIDINE | Brc1c(NC2=NCCN2)ccc2nccnc12 | pC25: 1.55 uM kg-1 |  |
| CANDESARTAN | CCOc1nc2cccc(C(=O)O)c2n1Cc1ccc(-c2ccccc2-c2nnn[nH]2)cc1 | T1/2: 9.0 hr |  |
| DIETHYLSTILBESTROL | CC/C(=C(/CC)c1ccc(O)cc1)c1ccc(O)cc1 | EC50: 9.0 nM |  |
| ACETAMINOPHEN | CC(=O)Nc1ccc(O)cc1 | Vdss: 0.95 L.kg-1 |  |
| AMPICILLIN | CC1(C)S[C@@H]2[C@H](NC(=O)[C@H](N)c3ccccc3)C(=O)N2[C@H]1C(=O)O | MIC: 5.0 ug.mL-1 |  |
| FLUNARIZINE | Fc1ccc(C(c2ccc(F)cc2)N2CCN(C/C=C/c3ccccc3)CC2)cc1 | IC50: 290.0 nM |  |
| FLOXURIDINE | O=c1[nH]c(=O)n([C@H]2C[C@H](O)[C@@H](CO)O2)cc1F | IC50: 2.0 nM |  |
| ETHYNODIOL DIACETATE | C#C[C@]1(OC(C)=O)CC[C@H]2[C@@H]3CCC4=C[C@@H](OC(C)=O)CC[C@@H]4[C@H]3CC[C@@]21C | Potency: 15848.9 nM |  |
| AMIFOSTINE | NCCCNCCSP(=O)(O)O | LD50: 1049.0 mg.kg-1 |  |
| ATROPINE | CN1[C@@H]2CC[C@H]1C[C@@H](OC(=O)C(CO)c1ccccc1)C2 | Ki: 0.45 nM |  |
| VALDECOXIB | Cc1onc(-c2ccccc2)c1-c1ccc(S(N)(=O)=O)cc1 | Ki: 54000.0 nM |  |
| ADENOSINE | Nc1ncnc2c1ncn2[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O | Km: 42000.0 nM |  |
| MIGLITOL | OCCN1C[C@H](O)[C@@H](O)[C@H](O)[C@H]1CO | Inhibition: 50.0 % | 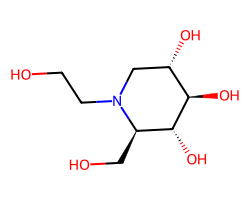 |
| ETHOXZOLAMIDE | CCOc1ccc2nc(S(N)(=O)=O)sc2c1 | Ki: 25.0 nM | 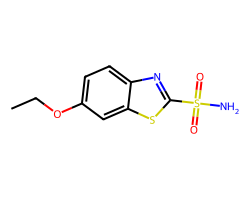 |
| FULVESTRANT | C[C@]12CC[C@@H]3c4ccc(O)cc4C[C@@H](CCCCCCCCC[S+]([O-])CCCC(F)(F)C(F)(F)F)[C@H]3[C@@H]1CC[C@@H]2O | IC50: 0.49 nM | 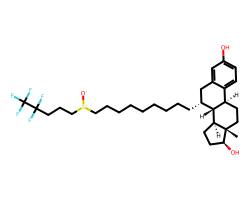 |
| CRIZOTINIB | C[C@@H](Oc1cc(-c2cnn(C3CCNCC3)c2)cnc1N)c1c(Cl)ccc(F)c1Cl | IC50: 8.0 nM | 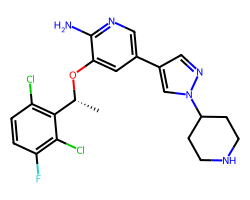 |
| ESTRADIOL | C[C@]12CC[C@@H]3c4ccc(O)cc4CC[C@H]3[C@@H]1CC[C@@H]2O | IC50: 1.5 nM | 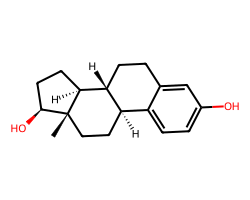 |
| DABIGATRAN | Cn1c(CNc2ccc(C(=N)N)cc2)nc2cc(C(=O)N(CCC(=O)O)c3ccccn3)ccc21 | IC50: 9.3 nM | 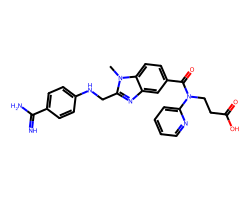 |
| OXATOMIDE | O=c1[nH]c2ccccc2n1CCCN1CCN(C(c2ccccc2)c2ccccc2)CC1 | A10: 0.014 mg l-1 | 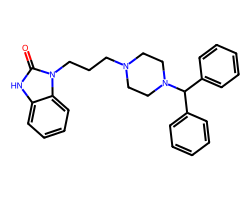 |
| BAZEDOXIFENE | Cc1c(-c2ccc(O)cc2)n(Cc2ccc(OCCN3CCCCCC3)cc2)c2ccc(O)cc12 | IC50: 23.0 nM | 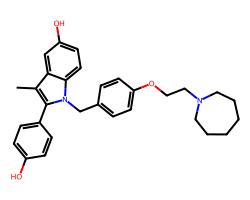 |
| ESTRONE | C[C@]12CC[C@@H]3c4ccc(O)cc4CC[C@H]3[C@@H]1CCC2=O | EC50: 1135.0 nM | 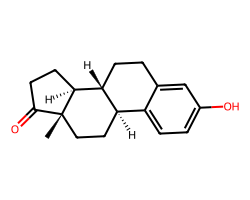 |
| PINACIDIL ANHYDROUS | CC(N/C(=N\C#N)Nc1ccncc1)C(C)(C)C | IC50: 800.0 nM | 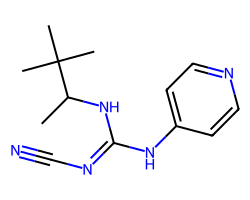 |
| METHAZOLAMIDE | CC(=O)/N=c1/sc(S(N)(=O)=O)nn1C | Ki: 50.0 nM | 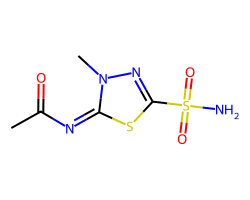 |
| PENTOSTATIN | OC[C@H]1O[C@@H](n2cnc3c2N=CNC[C@H]3O)C[C@@H]1O | Ki: 0.0025 nM | 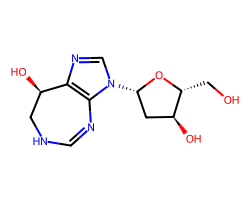 |
| MIBEFRADIL | COCC(=O)O[C@]1(CCN(C)CCCc2nc3ccccc3[nH]2)CCc2cc(F)ccc2[C@@H]1C(C)C | Inhibition: 86.0 % |  |
| MILRINONE | Cc1[nH]c(=O)c(C#N)cc1-c1ccncc1 | Mean ED50: 0.037 mg kg-1 |  |
Top Fragments
| SMILES | Avg pKi | Count | Structure |
|---|---|---|---|
| [3*]O[3*] | 6.96 | 263 | 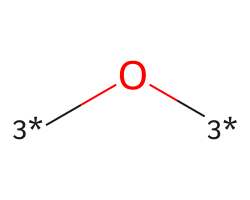 |
| [16*]c1ccccc1 | 5.73 | 186 | 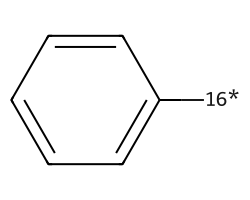 |
| [5*]N[5*] | 6.23 | 168 | 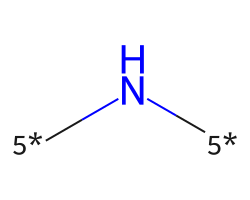 |
| [3*]OC | 6.19 | 154 | 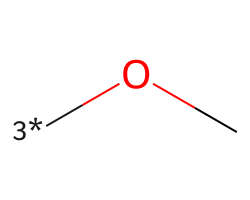 |
| [8*]CO | 8.01 | 122 | 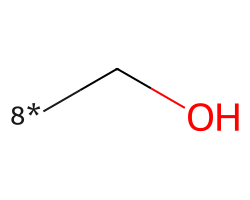 |