UDP-glucuronosyltransferase 2B7
Target ID: CHEMBL4370
Organism: Homo sapiens
Type: SINGLE PROTEIN
Molecules
| Name | SMILES | Bioactivity | Structure |
|---|---|---|---|
| 2-METHOXYESTRADIOL | COc1cc2c(cc1O)CC[C@@H]1[C@@H]2CC[C@]2(C)[C@@H](O)CC[C@@H]12 | GI50: 700.0 nM | 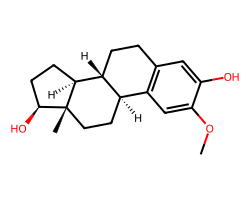 |
| HYDROXYPROGESTERONE | CC(=O)[C@@]1(O)CC[C@H]2[C@@H]3CCC4=CC(=O)CC[C@]4(C)[C@H]3CC[C@@]21C | Remaining activity: 89.0 % | 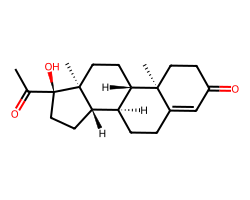 |
| ERGOCALCIFEROL | C=C1CC[C@H](O)C/C1=C/C=C1\CCC[C@]2(C)[C@@H]([C@H](C)/C=C/[C@H](C)C(C)C)CC[C@@H]12 | EC50: 3800.0 nM | 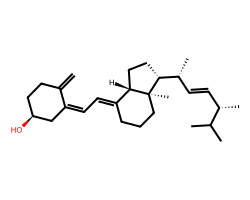 |
| EDARAVONE | CC1=NN(c2ccccc2)C(=O)C1 | PC50: 70.4 uM | 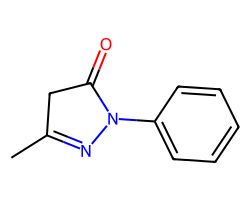 |
| ESTRONE | C[C@]12CC[C@@H]3c4ccc(O)cc4CC[C@H]3[C@@H]1CCC2=O | EC50: 1135.0 nM | 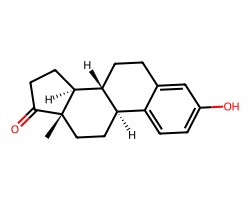 |
| DIETHYLSTILBESTROL | CC/C(=C(/CC)c1ccc(O)cc1)c1ccc(O)cc1 | EC50: 9.0 nM | 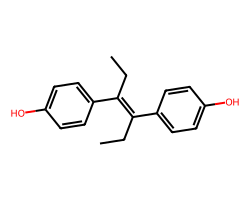 |
| ACETAMINOPHEN | CC(=O)Nc1ccc(O)cc1 | Vdss: 0.95 L.kg-1 | 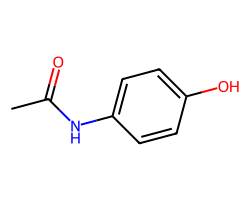 |
| ESTRADIOL | C[C@]12CC[C@@H]3c4ccc(O)cc4CC[C@H]3[C@@H]1CC[C@@H]2O | IC50: 1.5 nM | 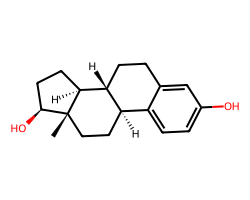 |
Top Fragments
| SMILES | Avg pKi | Count | Structure |
|---|---|---|---|
| c1ccc2c(c1)CC[C@@H]1[C@@H]2CCC2CCC[C@H]21 | 7.47 | 59 | 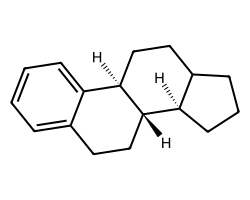 |
| [16*]c1ccc(O)cc1 | 8.05 | 42 | 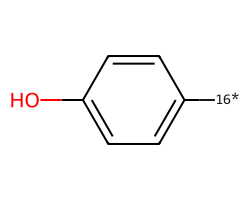 |
| [16*]c1cc2c(cc1O)CC[C@@H]1[C@@H]2CC[C@]2(C)[C@@H](O)CC[C@@H]12 | 6.15 | 30 | 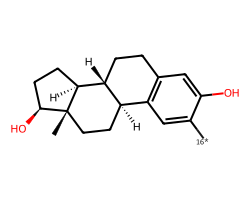 |
| [3*]OC | 6.15 | 30 | 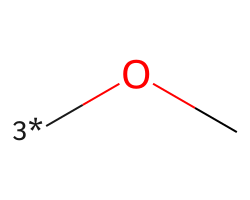 |
| [7*]C([8*])CC | 8.05 | 30 |  |