BEAS-2B
Target ID: CHEMBL4296403
Organism: Homo sapiens
Type: CELL-LINE
Molecules
| Name | SMILES | Bioactivity | Structure |
|---|---|---|---|
| VORINOSTAT | O=C(CCCCCCC(=O)Nc1ccccc1)NO | Inhibition: 100.0 % | 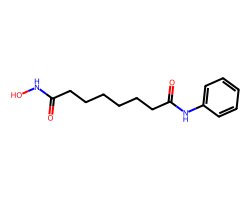 |
| AMPHOTERICIN B | C[C@@H]1[C@H](O)[C@@H](C)/C=C/C=C/C=C/C=C/C=C/C=C/C=C/[C@H](O[C@@H]2O[C@H](C)[C@@H](O)[C@H](N)[C@@H]2O)C[C@@H]2O[C@](O)(C[C@@H](O)C[C@@H](O)[C@H](O)CC[C@@H](O)C[C@@H](O)CC(=O)O[C@H]1C)C[C@H](O)[C@H]2C(=O)O | MIC: 4000.0 ug.mL-1 | 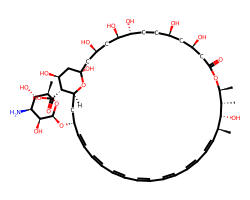 |
| FLUCONAZOLE | OC(Cn1cncn1)(Cn1cncn1)c1ccc(F)cc1F | IC80: 2.8 ug ml-1 | 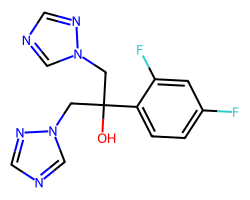 |
| SUNITINIB | CCN(CC)CCNC(=O)c1c(C)[nH]c(/C=C2\C(=O)Nc3ccc(F)cc32)c1C | IC50: 80.0 nM | 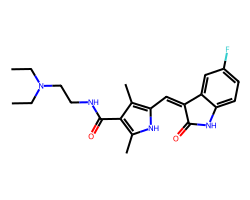 |
| SORAFENIB | CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(C(F)(F)F)c3)cc2)ccn1 | IC50: 12.0 nM | 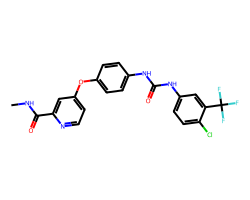 |
Top Fragments
| SMILES | Avg pKi | Count | Structure |
|---|---|---|---|
| [5*]N[5*] | 7.51 | 117 | 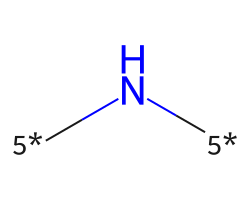 |
| [1*]C([6*])=O | 7.51 | 78 | 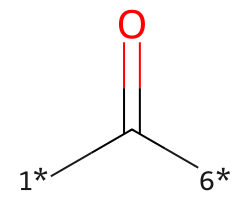 |
| [3*]O[3*] | 7.92 | 57 | 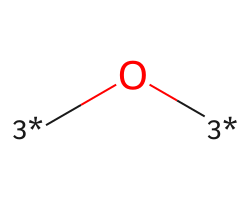 |
| [16*]c1ccccc1 | None | 39 | 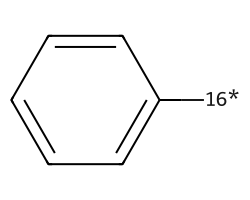 |
| [1*]C(=O)CCCCCCC(=O)NO | None | 39 | 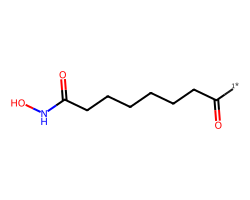 |