Thiosulfate sulfurtransferase
Target ID: CHEMBL4295835
Organism: Homo sapiens
Type: SINGLE PROTEIN
Molecules
| Name | SMILES | Bioactivity | Structure |
|---|---|---|---|
| HEXACHLOROPHENE | Oc1c(Cl)cc(Cl)c(Cl)c1Cc1c(O)c(Cl)cc(Cl)c1Cl | Control current: 237.4 % |  |
| GENTIAN VIOLET | CN(C)c1ccc(C(=C2C=CC(=[N+](C)C)C=C2)c2ccc(N(C)C)cc2)cc1.[Cl-] | Lysis: 100.0 % |  |
| ERGOCALCIFEROL | C=C1CC[C@H](O)C/C1=C/C=C1\CCC[C@]2(C)[C@@H]([C@H](C)/C=C/[C@H](C)C(C)C)CC[C@@H]12 | EC50: 3800.0 nM |  |
| ETHACRYNIC ACID | C=C(CC)C(=O)c1ccc(OCC(=O)O)c(Cl)c1Cl | mequiv of Na+/kg: 4.14 0-5h |  |
| CANDESARTAN | CCOc1nc2cccc(C(=O)O)c2n1Cc1ccc(-c2ccccc2-c2nnn[nH]2)cc1 | T1/2: 9.0 hr | 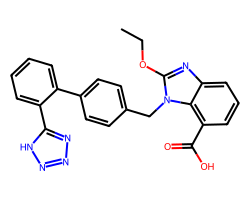 |
| AMPICILLIN | CC1(C)S[C@@H]2[C@H](NC(=O)[C@H](N)c3ccccc3)C(=O)N2[C@H]1C(=O)O | MIC: 5.0 ug.mL-1 | 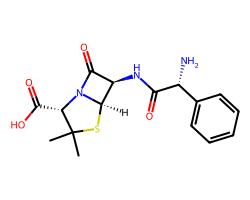 |
| BITHIONOL | Oc1c(Cl)cc(Cl)cc1Sc1cc(Cl)cc(Cl)c1O | Control current: 198.6 % | 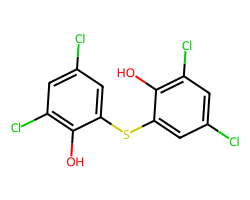 |
Top Fragments
| SMILES | Avg pKi | Count | Structure |
|---|---|---|---|
| [8*]C[8*] | None | 35 | 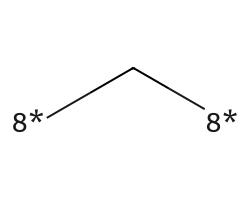 |
| [16*]c1ccc([16*])cc1 | None | 34 | 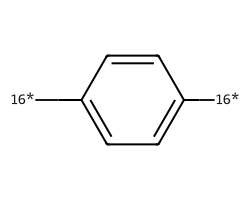 |
| [3*]O[3*] | None | 32 | 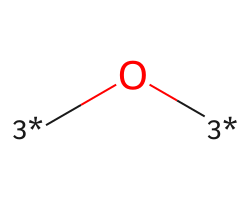 |
| c1ccc(Cc2ccccc2)cc1 | None | 23 | 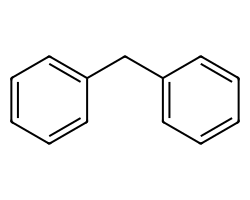 |
| [16*]c1c(O)c(Cl)cc(Cl)c1Cl | None | 23 | 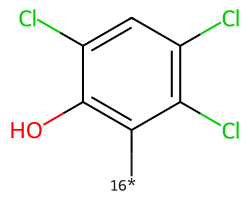 |