Carbonic anhydrase XIII
Target ID: CHEMBL3912
Organism: Homo sapiens
Type: SINGLE PROTEIN
Molecules
| Name | SMILES | Bioactivity | Structure |
|---|---|---|---|
| HISTIDINE | N[C@@H](Cc1c[nH]cn1)C(=O)O | KA: 4.0 uM |  |
| DORZOLAMIDE | CCN[C@H]1C[C@H](C)S(=O)(=O)c2sc(S(N)(=O)=O)cc21 | Ki: 50000.0 nM |  |
| HISTAMINE | NCCc1c[nH]cn1 | KA: 2.0 uM |  |
| ACETAZOLAMIDE | CC(=O)Nc1nnc(S(N)(=O)=O)s1 | Ki: 250.0 nM |  |
| TOPIRAMATE | CC1(C)O[C@@H]2[C@@H](CO[C@@]3(COS(N)(=O)=O)OC(C)(C)O[C@@H]23)O1 | Ki: 250.0 nM |  |
| DICHLORPHENAMIDE | NS(=O)(=O)c1cc(Cl)c(Cl)c(S(N)(=O)=O)c1 | Ki: 1200.0 nM |  |
| VALDECOXIB | Cc1onc(-c2ccccc2)c1-c1ccc(S(N)(=O)=O)cc1 | Ki: 54000.0 nM |  |
| SULFANILAMIDE | Nc1ccc(S(N)(=O)=O)cc1 | Ki: 28000.0 nM |  |
| PHENYLALANINE | N[C@@H](Cc1ccccc1)C(=O)O | Solubility: 245000.0 ug.mL-1 | 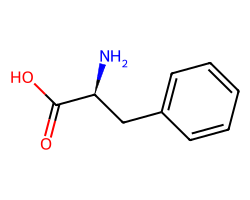 |
| ETHOXZOLAMIDE | CCOc1ccc2nc(S(N)(=O)=O)sc2c1 | Ki: 25.0 nM | 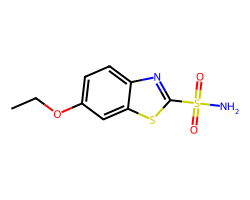 |
| BRINZOLAMIDE | CCN[C@H]1CN(CCCOC)S(=O)(=O)c2sc(S(N)(=O)=O)cc21 | Ki: 37.0 nM | 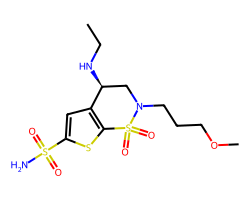 |
| BORTEZOMIB | CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)c1cnccn1)B(O)O | Ki: 0.62 nM | 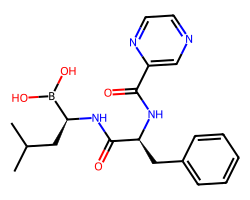 |
| METHAZOLAMIDE | CC(=O)/N=c1/sc(S(N)(=O)=O)nn1C | Ki: 50.0 nM | 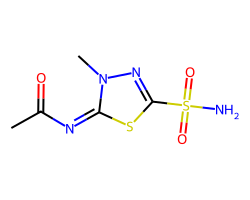 |
Top Fragments
| SMILES | Avg pKi | Count | Structure |
|---|---|---|---|
| [16*]c1ccccc1 | 6.91 | 107 | 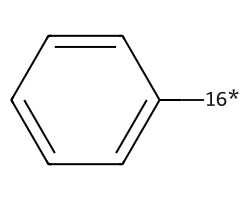 |
| c1ccccc1 | 5.25 | 103 | 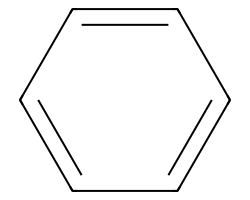 |
| [4*]CC | 6.38 | 102 | 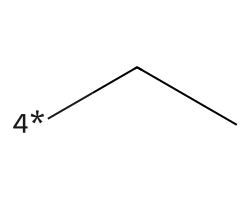 |
| [5*]N[5*] | 7.98 | 74 | 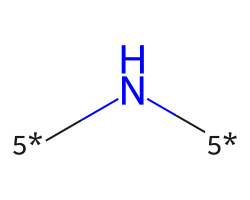 |
| [14*]c1c[nH]cn1 | None | 72 | 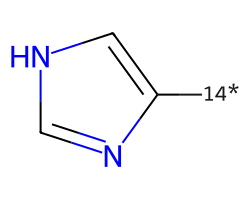 |