MAP kinase ERK1
Target ID: CHEMBL3385
Organism: Homo sapiens
Type: SINGLE PROTEIN
Molecules
| Name | SMILES | Bioactivity | Structure |
|---|---|---|---|
| ABAMECTIN | CC[C@H](C)[C@H]1O[C@]2(C=C[C@@H]1C)C[C@@H]1C[C@@H](C/C=C(\C)[C@@H](O[C@H]3C[C@H](OC)[C@@H](O[C@H]4C[C@H](OC)[C@@H](O)[C@H](C)O4)[C@H](C)O3)[C@@H](C)/C=C/C=C3\CO[C@@H]4[C@H](O)C(C)=C[C@@H](C(=O)O1)[C@]34O)O2.CO[C@H]1C[C@H](O[C@H]2[C@H](C)O[C@@H](O[C@@H]3/C(C)=C/C[C@@H]4C[C@@H](C[C@]5(C=C[C@H](C)[C@@H](C(C)C)O5)O4)OC(=O)[C@@H]4C=C(C)[C@@H](O)[C@H]5OC/C(=C\C=C\[C@@H]3C)[C@]54O)C[C@@H]2OC)O[C@@H](C)[C@@H]1O | EC50: 12000.0 nM |  |
| GENISTEIN | O=c1c(-c2ccc(O)cc2)coc2cc(O)cc(O)c12 | IC50: 1000.0 nM |  |
| FLUOCINOLONE ACETONIDE | CC1(C)O[C@@H]2C[C@H]3[C@@H]4C[C@H](F)C5=CC(=O)C=C[C@]5(C)[C@@]4(F)[C@@H](O)C[C@]3(C)[C@]2(C(=O)CO)O1 | Potency: 35481.3 nM |  |
| INAMRINONE | Nc1cc(-c2ccncc2)c[nH]c1=O | Mean ED50: 0.389 mg kg-1 |  |
| TANDUTINIB | COc1cc2c(N3CCN(C(=O)Nc4ccc(OC(C)C)cc4)CC3)ncnc2cc1OCCCN1CCCCC1 | IC50: 26.0 nM | 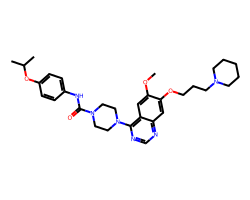 |
| AMINOPHYLLINE | Cn1c(=O)c2[nH]cnc2n(C)c1=O.Cn1c(=O)c2[nH]cnc2n(C)c1=O.NCCN | IC50: 16700.0 nM | 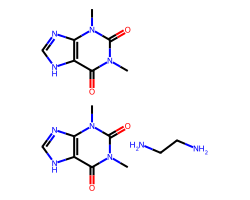 |
| 6-METHOXY-2-NAPHTHYLACETIC ACID | COc1ccc2cc(CC(=O)O)ccc2c1 | Inhibition: 5.0 % | 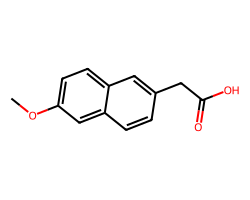 |
| BOSUTINIB | COc1cc(Nc2c(C#N)cnc3cc(OCCCN4CCN(C)CC4)c(OC)cc23)c(Cl)cc1Cl | IC50: 1.3 nM | 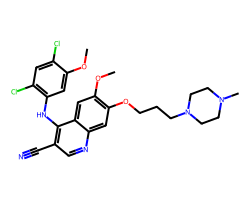 |
| VANDETANIB | COc1cc2/c(=N/c3ccc(Br)cc3F)nc[nH]c2cc1OCC1CCN(C)CC1 | IC50: 900.0 nM | 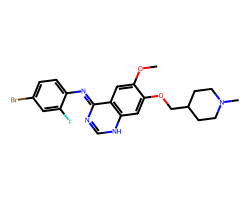 |
| ERLOTINIB | C#Cc1cccc(Nc2ncnc3cc(OCCOC)c(OCCOC)cc23)c1 | IC50: 1450.0 nM | 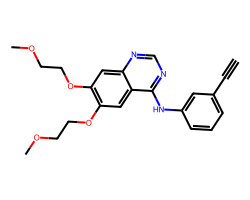 |
| RAF-265 | Cn1c(Nc2ccc(C(F)(F)F)cc2)nc2cc(Oc3ccnc(-c4ncc(C(F)(F)F)[nH]4)c3)ccc21 | Kd: 10000.0 nM | 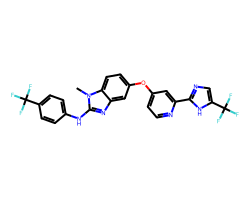 |
| BUFLOMEDIL | COc1cc(OC)c(C(=O)CCCN2CCCC2)c(OC)c1 | Ki: 60.0 nM | 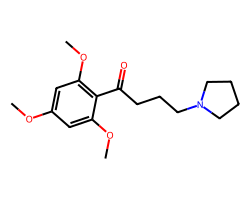 |
| AMITRAZ | Cc1ccc(/N=C/N(C)/C=N/c2ccc(C)cc2C)c(C)c1 | Potency: 0.9 nM |  |
| AMPRENAVIR | CC(C)CN(C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)O[C@H]1CCOC1)S(=O)(=O)c1ccc(N)cc1 | Ki: 0.16 nM |  |
| AMITRIPTYLINE | CN(C)CCC=C1c2ccccc2CCc2ccccc21 | IC50: 61.0 nM |  |
| AMPHOTERICIN B | C[C@@H]1[C@H](O)[C@@H](C)/C=C/C=C/C=C/C=C/C=C/C=C/C=C/[C@H](O[C@@H]2O[C@H](C)[C@@H](O)[C@H](N)[C@@H]2O)C[C@@H]2O[C@](O)(C[C@@H](O)C[C@@H](O)[C@H](O)CC[C@@H](O)C[C@@H](O)CC(=O)O[C@H]1C)C[C@H](O)[C@H]2C(=O)O | MIC: 4000.0 ug.mL-1 |  |
| ACETAZOLAMIDE | CC(=O)Nc1nnc(S(N)(=O)=O)s1 | Ki: 250.0 nM | 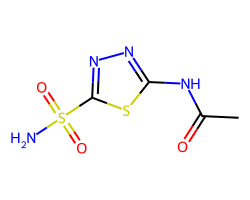 |
| CANTHARIDIN | C[C@@]12C(=O)OC(=O)[C@]1(C)[C@H]1CC[C@@H]2O1 | IC50: 160.0 nM | 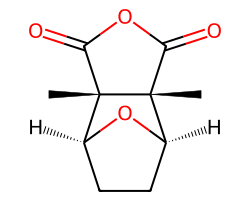 |
| ATENOLOL | CC(C)NCC(O)COc1ccc(CC(N)=O)cc1 | Pc: 1000000.0 cm s-1 | 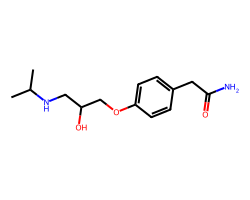 |
| CARBIMAZOLE | CCOC(=O)n1ccn(C)c1=S | IC50: 10400.0 nM | 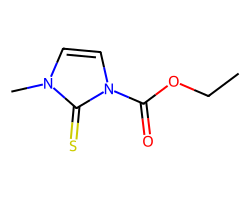 |
| ACLARUBICIN | CC[C@@]1(O)C[C@H](O[C@H]2C[C@H](N(C)C)[C@H](O[C@H]3C[C@H](O)[C@H](O[C@H]4CCC(=O)[C@H](C)O4)[C@H](C)O3)[C@H](C)O2)c2c(cc3c(c2O)C(=O)c2c(O)cccc2C3=O)[C@H]1C(=O)OC | IC50: 9900.0 nM |  |
| ANISINDIONE | COc1ccc(C2C(=O)c3ccccc3C2=O)cc1 | Inhibition: 1.3 % |  |
| AMINOCAPROIC ACID | NCCCCCC(=O)O | Ki: 50000.0 nM |  |
| BETADEX | OC[C@H]1O[C@@H]2O[C@H]3[C@H](O)[C@@H](O)[C@@H](O[C@H]4[C@H](O)[C@@H](O)[C@@H](O[C@H]5[C@H](O)[C@@H](O)[C@@H](O[C@H]6[C@H](O)[C@@H](O)[C@@H](O[C@H]7[C@H](O)[C@@H](O)[C@@H](O[C@H]8[C@H](O)[C@@H](O)[C@@H](O[C@H]1[C@H](O)[C@H]2O)O[C@@H]8CO)O[C@@H]7CO)O[C@@H]6CO)O[C@@H]5CO)O[C@@H]4CO)O[C@@H]3CO | k_obs: 4.16e-07 s-1 |  |
| BRIMONIDINE | Brc1c(NC2=NCCN2)ccc2nccnc12 | pC25: 1.55 uM kg-1 | 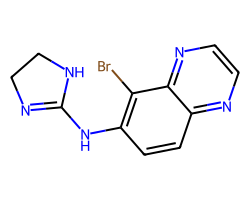 |
| FLUCONAZOLE | OC(Cn1cncn1)(Cn1cncn1)c1ccc(F)cc1F | IC80: 2.8 ug ml-1 | 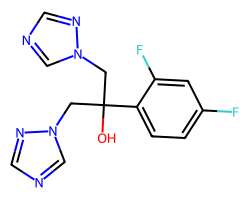 |
| AMIKACIN | NCC[C@H](O)C(=O)N[C@@H]1C[C@H](N)[C@@H](O[C@H]2O[C@H](CN)[C@@H](O)[C@H](O)[C@H]2O)[C@H](O)[C@H]1O[C@H]1O[C@H](CO)[C@@H](O)[C@H](N)[C@H]1O | C50: 0.21 uM | 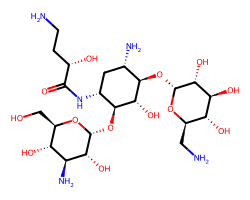 |
| AST-487 | CCN1CCN(Cc2ccc(NC(=O)Nc3ccc(Oc4cc(NC)ncn4)cc3)cc2C(F)(F)F)CC1 | Kd: 2.3 nM | 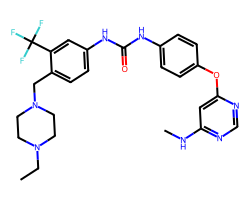 |
| RUBOXISTAURIN | CN(C)C[C@@H]1CCn2cc(c3ccccc32)C2=C(C(=O)NC2=O)c2cn(c3ccccc23)CCO1 | IC50: 300.0 nM |  |
| CANDESARTAN | CCOc1nc2cccc(C(=O)O)c2n1Cc1ccc(-c2ccccc2-c2nnn[nH]2)cc1 | T1/2: 9.0 hr |  |
| ACETAMINOPHEN | CC(=O)Nc1ccc(O)cc1 | Vdss: 0.95 L.kg-1 |  |
| EUCALYPTOL | CC12CCC(CC1)C(C)(C)O2 | MIC: 800.0 ug.mL-1 |  |
| HALOPROGIN | Clc1cc(Cl)c(OCC#CI)cc1Cl | MIC: 3.13 ug.mL-1 | 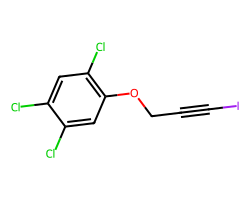 |
| AMPICILLIN | CC1(C)S[C@@H]2[C@H](NC(=O)[C@H](N)c3ccccc3)C(=O)N2[C@H]1C(=O)O | MIC: 5.0 ug.mL-1 | 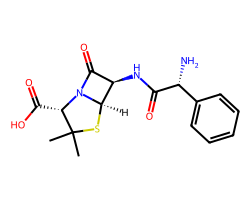 |
| CP-724714 | COCC(=O)NC/C=C/c1ccc2ncnc(Nc3ccc(Oc4ccc(C)nc4)c(C)c3)c2c1 | IC50: 12.0 nM | 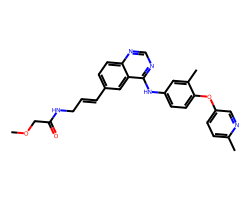 |
| ALVOCIDIB | CN1CC[C@H](c2c(O)cc(O)c3c(=O)cc(-c4ccccc4Cl)oc23)[C@H](O)C1 | IC50: 330.0 nM | 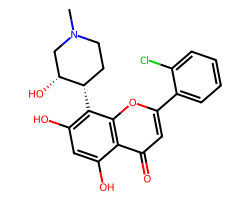 |
| AZARIBINE | CC(=O)OC[C@H]1O[C@@H](n2ncc(=O)[nH]c2=O)[C@H](OC(C)=O)[C@@H]1OC(C)=O | Potency: 562.3 nM | 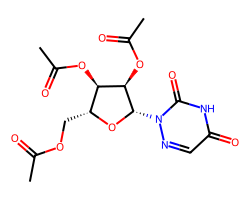 |
| ACEBUTOLOL | CCCC(=O)Nc1ccc(OCC(O)CNC(C)C)c(C(C)=O)c1 | LD50: 76.0 mg.kg-1 | 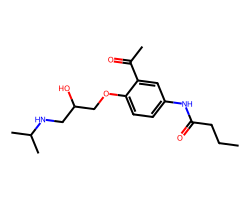 |
| ASTROMICIN | CO[C@H]1[C@@H](O)[C@H](N)[C@@H](O[C@H]2O[C@H]([C@H](C)N)CC[C@H]2N)[C@H](O)[C@@H]1N(C)C(=O)CN | Hepatotoxicity (moderate): 7.3 % | 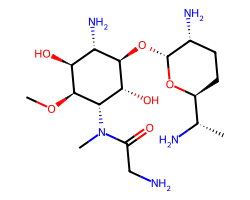 |
| BITHIONOL | Oc1c(Cl)cc(Cl)cc1Sc1cc(Cl)cc(Cl)c1O | Control current: 198.6 % | 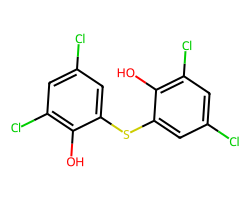 |
| FLOXURIDINE | O=c1[nH]c(=O)n([C@H]2C[C@H](O)[C@@H](CO)O2)cc1F | IC50: 2.0 nM | 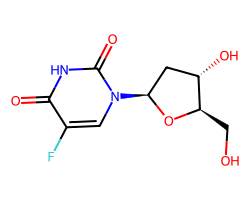 |
| BETAMETHASONE PHOSPHORIC ACID | C[C@H]1C[C@H]2[C@@H]3CCC4=CC(=O)C=C[C@]4(C)[C@@]3(F)[C@@H](O)C[C@]2(C)[C@@]1(O)C(=O)COP(=O)(O)O | Inhibition: 96.0 % | 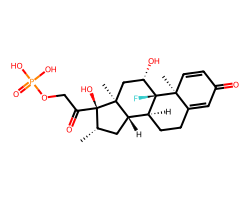 |
| AMOXICILLIN | CC1(C)S[C@@H]2[C@H](NC(=O)[C@H](N)c3ccc(O)cc3)C(=O)N2[C@H]1C(=O)O | MIC: 0.021 ug.mL-1 | 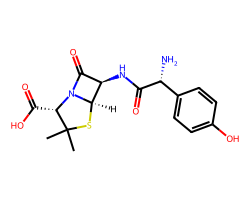 |
| ACONITINE | CCN1C[C@]2(COC)[C@H](O)C[C@H](OC)[C@@]34[C@@H]5C[C@@]6(O)[C@H](OC(=O)c7ccccc7)[C@@H]5[C@@](OC(C)=O)([C@@H]([C@H](OC)[C@H]23)[C@@H]14)[C@@H](O)[C@@H]6OC | Activity: 160.0 bpm | 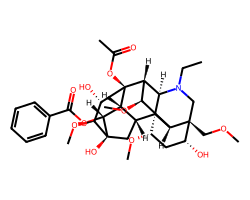 |
| AMIFOSTINE | NCCCNCCSP(=O)(O)O | LD50: 1049.0 mg.kg-1 | 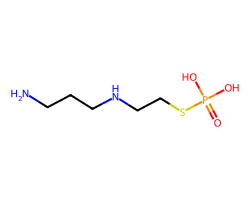 |
| ASTEMIZOLE | COc1ccc(CCN2CCC(Nc3nc4ccccc4n3Cc3ccc(F)cc3)CC2)cc1 | Binding energy: 11.3 kCal mol-1 | 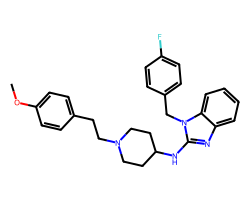 |
| CAFFEINE | Cn1c(=O)c2c(ncn2C)n(C)c1=O | Vdss: 0.61 L.kg-1 | 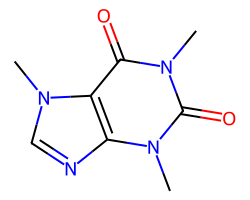 |
| ATROPINE | CN1[C@@H]2CC[C@H]1C[C@@H](OC(=O)C(CO)c1ccccc1)C2 | Ki: 0.45 nM | 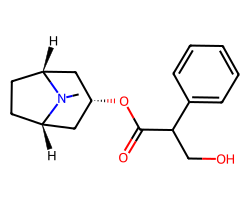 |
| MOTESANIB | CC1(C)CNc2cc(NC(=O)c3cccnc3NCc3ccncc3)ccc21 | Kd: 1100.0 nM | 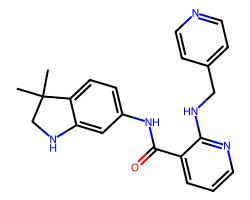 |
| SUNITINIB | CCN(CC)CCNC(=O)c1c(C)[nH]c(/C=C2\C(=O)Nc3ccc(F)cc32)c1C | IC50: 80.0 nM | 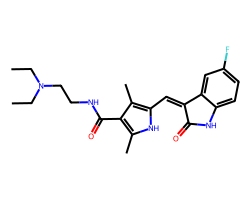 |
| AZD-1152-HQPA | CCN(CCO)CCCOc1ccc2c(Nc3cc(CC(=O)Nc4cccc(F)c4)[nH]n3)ncnc2c1 | Ki: 10000.0 nM | 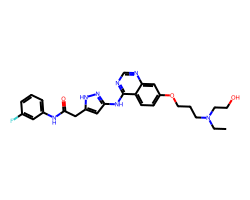 |
| QUERCETIN | O=c1c(O)c(-c2ccc(O)c(O)c2)oc2cc(O)cc(O)c12 | IC50: 32870.0 nM | 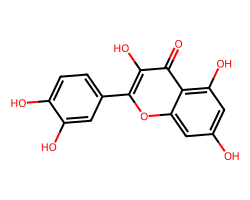 |
| AMILORIDE | N=C(N)NC(=O)c1nc(Cl)c(N)nc1N | Pc: 780000.0 cm s-1 | 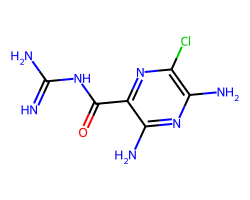 |
| AT-7519 | O=C(NC1CCNCC1)c1n[nH]cc1NC(=O)c1c(Cl)cccc1Cl | IC50: 47.0 nM | 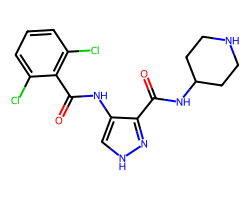 |
| LINIFANIB | Cc1ccc(F)c(NC(=O)Nc2ccc(-c3cccc4[nH]nc(N)c34)cc2)c1 | IC50: 4.0 nM | 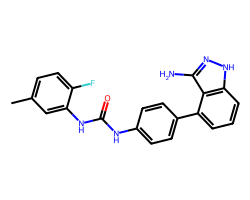 |
| LAPATINIB | CS(=O)(=O)CCNCc1ccc(-c2ccc3ncnc(Nc4ccc(OCc5cccc(F)c5)c(Cl)c4)c3c2)o1 | IC50: 10.0 nM | 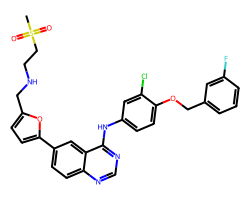 |
| DOVITINIB | CN1CCN(c2ccc3nc(-c4c(N)c5c(F)cccc5[nH]c4=O)[nH]c3c2)CC1 | IC50: 65.0 nM | 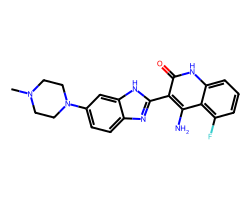 |
| TOFACITINIB | C[C@@H]1CCN(C(=O)CC#N)C[C@@H]1N(C)c1ncnc2[nH]ccc12 | IC50: 13.0 nM | 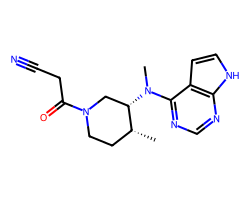 |
| CANERTINIB | C=CC(=O)Nc1cc2c(Nc3ccc(F)c(Cl)c3)ncnc2cc1OCCCN1CCOCC1 | IC50: 74.0 nM | 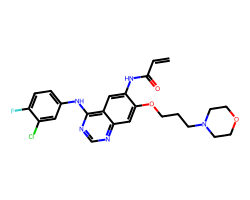 |
| VATALANIB | Clc1ccc(Nc2nnc(Cc3ccncc3)c3ccccc23)cc1 | IC50: 37.0 nM | 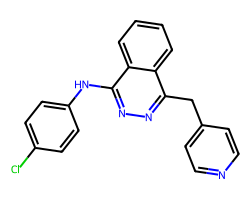 |
| BMS-387032 | CC(C)(C)c1cnc(CSc2cnc(NC(=O)C3CCNCC3)s2)o1 | IC50: 48000.0 nM |  |
| PAZOPANIB | Cc1ccc(Nc2nccc(N(C)c3ccc4c(C)n(C)nc4c3)n2)cc1S(N)(=O)=O | IC50: 10.0 nM |  |
| CRIZOTINIB | C[C@@H](Oc1cc(-c2cnn(C3CCNCC3)c2)cnc1N)c1c(Cl)ccc(F)c1Cl | IC50: 8.0 nM |  |
| AMINOPTERIN | Nc1nc(N)c2nc(CNc3ccc(C(=O)N[C@@H](CCC(=O)O)C(=O)O)cc3)cnc2n1 | Ki: 0.0037 nM |  |
| ESTRADIOL | C[C@]12CC[C@@H]3c4ccc(O)cc4CC[C@H]3[C@@H]1CC[C@@H]2O | IC50: 1.5 nM | 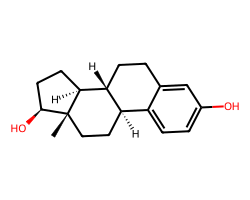 |
| IMATINIB | Cc1ccc(NC(=O)c2ccc(CN3CCN(C)CC3)cc2)cc1Nc1nccc(-c2cccnc2)n1 | IC50: 40.0 nM | 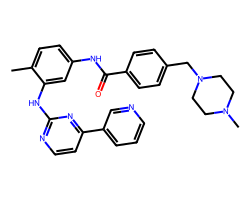 |
| GEFITINIB | COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1OCCCN1CCOCC1 | IC50: 515.0 nM | 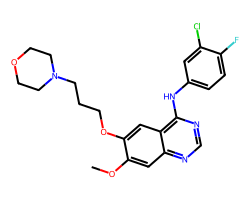 |
| MLN-8054 | O=C(O)c1ccc(Nc2ncc3c(n2)-c2ccc(Cl)cc2C(c2c(F)cccc2F)=NC3)cc1 | Activity: 39.7 % | 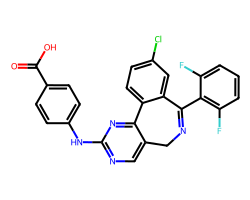 |
| DORAMAPIMOD | Cc1ccc(-n2nc(C(C)(C)C)cc2NC(=O)Nc2ccc(OCCN3CCOCC3)c3ccccc23)cc1 | Kd: 0.046 nM | 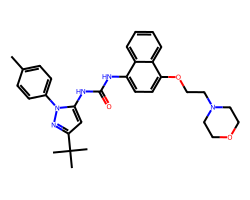 |
| TOZASERTIB | Cc1cc(Nc2cc(N3CCN(C)CC3)nc(Sc3ccc(NC(=O)C4CC4)cc3)n2)n[nH]1 | Ki: 0.6 nM | 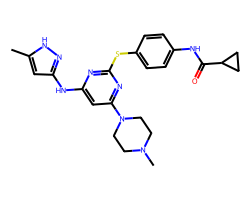 |
| SELICICLIB | CC[C@H](CO)Nc1nc(NCc2ccccc2)c2ncn(C(C)C)c2n1 | IC50: 450.0 nM | 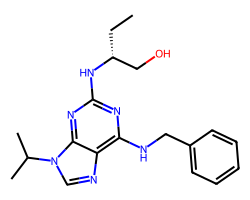 |
| PELITINIB | CCOc1cc2ncc(C#N)c(Nc3ccc(F)c(Cl)c3)c2cc1NC(=O)/C=C/CN(C)C | IC50: 83.0 nM | 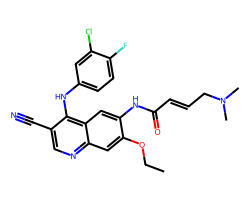 |
| MIDOSTAURIN | CO[C@@H]1[C@H](N(C)C(=O)c2ccccc2)C[C@H]2O[C@]1(C)n1c3ccccc3c3c4c(c5c6ccccc6n2c5c31)C(=O)NC4 | Kd: 11.0 nM | 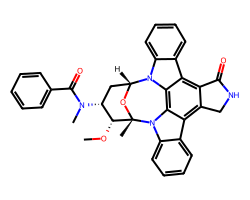 |
| SORAFENIB | CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(C(F)(F)F)c3)cc2)ccn1 | IC50: 12.0 nM | 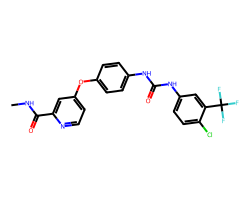 |
| SU-014813 | Cc1[nH]c(/C=C2\C(=O)Nc3ccc(F)cc32)c(C)c1C(=O)NC[C@H](O)CN1CCOCC1 | Kd: 1.8 nM | 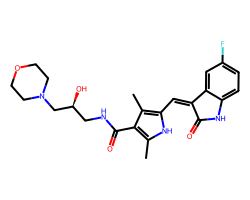 |
| NEFLAMAPIMOD | O=c1ncn2nc(Sc3ccc(F)cc3F)ccc2c1-c1c(Cl)cccc1Cl | IC50: 22.0 nM | 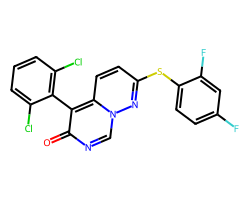 |
Top Fragments
| SMILES | Avg pKi | Count | Structure |
|---|---|---|---|
| [5*]N[5*] | 7.5 | 1148 | 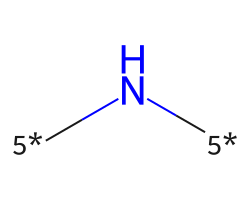 |
| [3*]O[3*] | 7.19 | 770 | 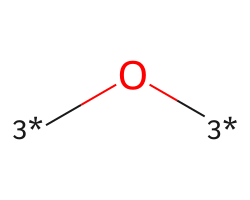 |
| [1*]C([6*])=O | 7.74 | 434 | 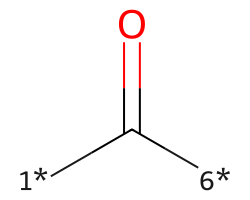 |
| [16*]c1ccc([16*])cc1 | 8.2 | 400 | 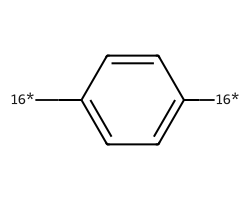 |
| [3*]OC | 6.95 | 390 | 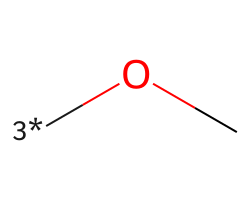 |