Estrogen receptor beta
Target ID: CHEMBL242
Organism: Homo sapiens
Type: SINGLE PROTEIN
Molecules
| Name | SMILES | Bioactivity | Structure |
|---|---|---|---|
| GENISTEIN | O=c1c(-c2ccc(O)cc2)coc2cc(O)cc(O)c12 | IC50: 1000.0 nM | 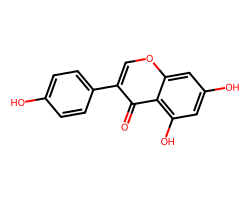 |
| MIFEPRISTONE | CC#C[C@]1(O)CC[C@H]2[C@@H]3CCC4=CC(=O)CCC4=C3[C@@H](c3ccc(N(C)C)cc3)C[C@@]21C | IC50: 0.028 nM | 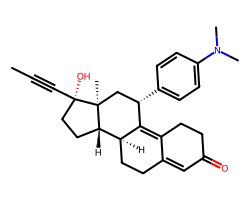 |
| DIETHYLSTILBESTROL | CC/C(=C(/CC)c1ccc(O)cc1)c1ccc(O)cc1 | EC50: 9.0 nM | 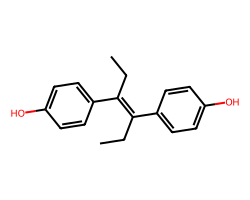 |
| 2-METHOXYESTRADIOL | COc1cc2c(cc1O)CC[C@@H]1[C@@H]2CC[C@]2(C)[C@@H](O)CC[C@@H]12 | GI50: 700.0 nM | 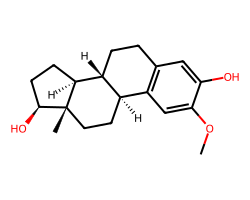 |
| ALFATRADIOL | C[C@]12CC[C@@H]3c4ccc(O)cc4CC[C@H]3[C@@H]1CC[C@H]2O | EC50: 1230.0 nM | 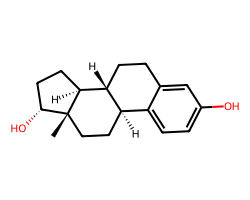 |
| PIPENDOXIFENE | Cc1c(-c2ccc(O)cc2)n(Cc2ccc(OCCN3CCCCC3)cc2)c2ccc(O)cc12 | IC50: 14.0 nM | 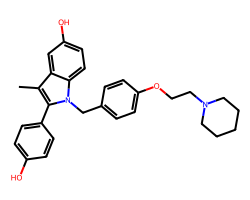 |
| DAIDZEIN | O=c1c(-c2ccc(O)cc2)coc2cc(O)ccc12 | Suppression: 22.0 % | 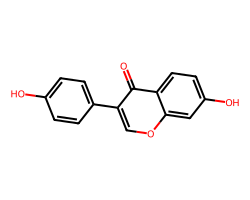 |
| FULVESTRANT | C[C@]12CC[C@@H]3c4ccc(O)cc4C[C@@H](CCCCCCCCC[S+]([O-])CCCC(F)(F)C(F)(F)F)[C@H]3[C@@H]1CC[C@@H]2O | IC50: 0.49 nM | 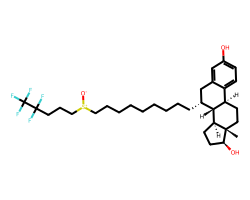 |
| RALOXIFENE HYDROCHLORIDE | Cl.O=C(c1ccc(OCCN2CCCCC2)cc1)c1c(-c2ccc(O)cc2)sc2cc(O)ccc12 | IC50: 0.4 nM | 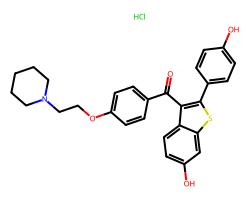 |
| ESTRADIOL | C[C@]12CC[C@@H]3c4ccc(O)cc4CC[C@H]3[C@@H]1CC[C@@H]2O | IC50: 1.5 nM | 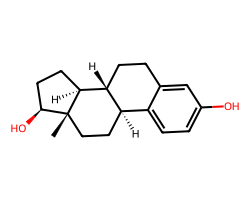 |
| TAMOXIFEN | CC/C(=C(\c1ccccc1)c1ccc(OCCN(C)C)cc1)c1ccccc1 | IC50: 6750.0 nM | 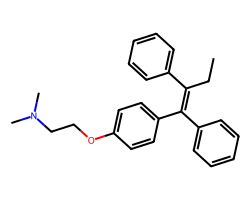 |
| BAZEDOXIFENE | Cc1c(-c2ccc(O)cc2)n(Cc2ccc(OCCN3CCCCCC3)cc2)c2ccc(O)cc12 | IC50: 23.0 nM | 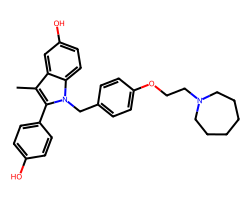 |
| ESTRONE | C[C@]12CC[C@@H]3c4ccc(O)cc4CC[C@H]3[C@@H]1CCC2=O | EC50: 1135.0 nM | 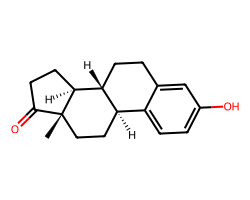 |
| RALOXIFENE | O=C(c1ccc(OCCN2CCCCC2)cc1)c1c(-c2ccc(O)cc2)sc2cc(O)ccc12 | IC50: 1.8 nM | 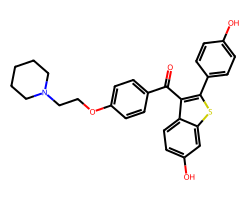 |
| CYCLOFENIL | CC(=O)Oc1ccc(C(=C2CCCCC2)c2ccc(OC(C)=O)cc2)cc1 | Potency: 28183.8 nM | 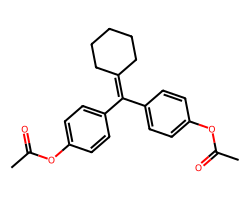 |
Top Fragments
| SMILES | Avg pKi | Count | Structure |
|---|---|---|---|
| [16*]c1ccc(O)cc1 | 7.83 | 212 | 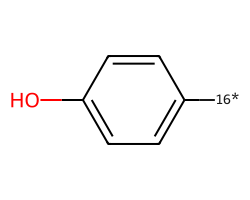 |
| [16*]c1ccc([16*])cc1 | 7.76 | 200 | 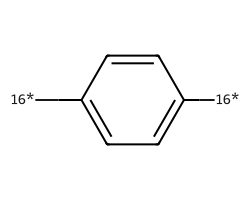 |
| [3*]O[3*] | 7.25 | 169 | 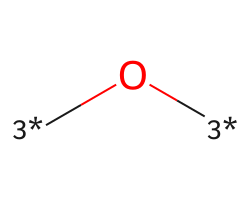 |
| [4*]CC[4*] | 7.77 | 142 | 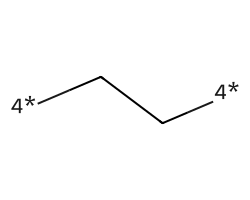 |
| c1ccc2c(c1)CC[C@@H]1[C@@H]2CCC2CCC[C@H]21 | 7.52 | 118 | 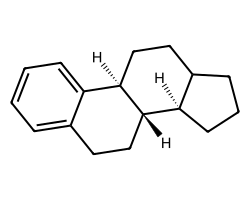 |