MOLT-13
Target ID: CHEMBL2366103
Organism: Homo sapiens
Type: CELL-LINE
Molecules
| Name | SMILES | Bioactivity | Structure |
|---|---|---|---|
| BOSUTINIB | COc1cc(Nc2c(C#N)cnc3cc(OCCCN4CCN(C)CC4)c(OC)cc23)c(Cl)cc1Cl | IC50: 1.3 nM | 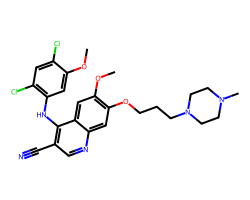 |
| SORAFENIB | CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(C(F)(F)F)c3)cc2)ccn1 | IC50: 12.0 nM | 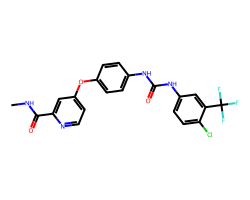 |
| SARACATINIB | CN1CCN(CCOc2cc(OC3CCOCC3)c3c(Nc4c(Cl)ccc5c4OCO5)ncnc3c2)CC1 | IC50: 2.7 nM | 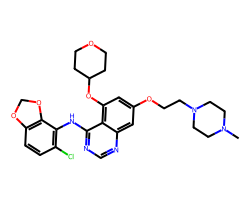 |
| VORINOSTAT | O=C(CCCCCCC(=O)Nc1ccccc1)NO | Inhibition: 100.0 % | 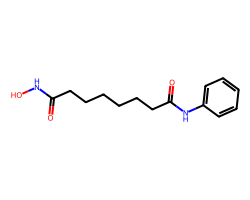 |
| PYRIMETHAMINE | CCc1nc(N)nc(N)c1-c1ccc(Cl)cc1 | IC50: 2800.0 nM | 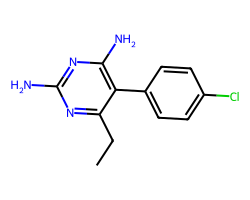 |
| DORAMAPIMOD | Cc1ccc(-n2nc(C(C)(C)C)cc2NC(=O)Nc2ccc(OCCN3CCOCC3)c3ccccc23)cc1 | Kd: 0.046 nM | 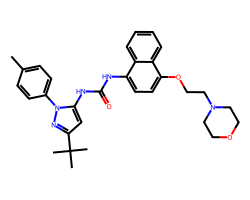 |
| MOTESANIB | CC1(C)CNc2cc(NC(=O)c3cccnc3NCc3ccncc3)ccc21 | Kd: 1100.0 nM | 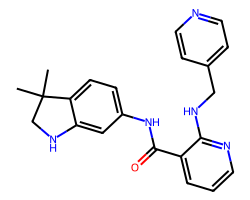 |
| IMATINIB | Cc1ccc(NC(=O)c2ccc(CN3CCN(C)CC3)cc2)cc1Nc1nccc(-c2cccnc2)n1 | IC50: 40.0 nM | 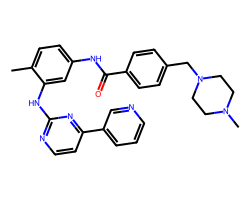 |
| GEFITINIB | COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1OCCCN1CCOCC1 | IC50: 515.0 nM | 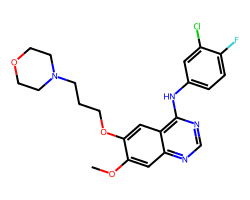 |
Top Fragments
| SMILES | Avg pKi | Count | Structure |
|---|---|---|---|
| [5*]N[5*] | 7.91 | 312 | 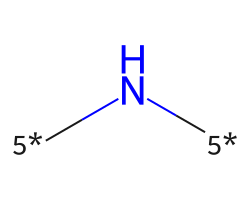 |
| [3*]O[3*] | 8.4 | 195 | 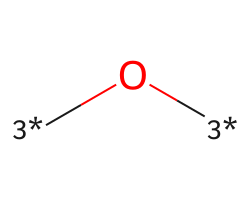 |
| [5*]N1CCN(C)CC1 | 8.28 | 117 | 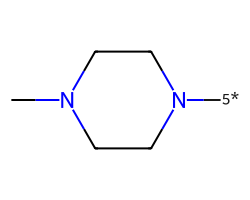 |
| [1*]C([6*])=O | 7.09 | 117 | 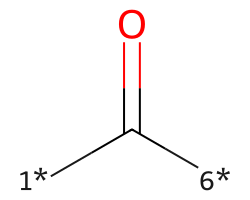 |
| [4*]CCC[4*] | 7.59 | 78 | 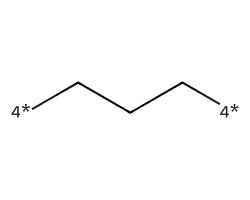 |