Motilin receptor
Target ID: CHEMBL2203
Organism: Homo sapiens
Type: SINGLE PROTEIN
Molecules
| Name | SMILES | Bioactivity | Structure |
|---|---|---|---|
| METHOTREXATE | CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(C(=O)N[C@@H](CCC(=O)O)C(=O)O)cc1 | ID50: 6.2 nM |  |
| FUROSEMIDE | NS(=O)(=O)c1cc(C(=O)O)c(NCc2ccco2)cc1Cl | Ki: 100000.0 nM |  |
| FLUVASTATIN | CC(C)n1c(/C=C/C(O)CC(O)CC(=O)O)c(-c2ccc(F)cc2)c2ccccc21 | IC50: 28.0 nM |  |
| ETHACRYNIC ACID | C=C(CC)C(=O)c1ccc(OCC(=O)O)c(Cl)c1Cl | mequiv of Na+/kg: 4.14 0-5h |  |
| BOSUTINIB | COc1cc(Nc2c(C#N)cnc3cc(OCCCN4CCN(C)CC4)c(OC)cc23)c(Cl)cc1Cl | IC50: 1.3 nM |  |
| ERLOTINIB | C#Cc1cccc(Nc2ncnc3cc(OCCOC)c(OCCOC)cc23)c1 | IC50: 1450.0 nM |  |
| AMITRIPTYLINE | CN(C)CCC=C1c2ccccc2CCc2ccccc21 | IC50: 61.0 nM |  |
| AMPHOTERICIN B | C[C@@H]1[C@H](O)[C@@H](C)/C=C/C=C/C=C/C=C/C=C/C=C/C=C/[C@H](O[C@@H]2O[C@H](C)[C@@H](O)[C@H](N)[C@@H]2O)C[C@@H]2O[C@](O)(C[C@@H](O)C[C@@H](O)[C@H](O)CC[C@@H](O)C[C@@H](O)CC(=O)O[C@H]1C)C[C@H](O)[C@H]2C(=O)O | MIC: 4000.0 ug.mL-1 |  |
| ATENOLOL | CC(C)NCC(O)COc1ccc(CC(N)=O)cc1 | Pc: 1000000.0 cm s-1 | 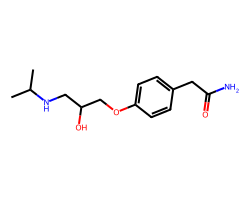 |
| EMETINE | CC[C@H]1CN2CCc3cc(OC)c(OC)cc3[C@@H]2C[C@@H]1C[C@H]1NCCc2cc(OC)c(OC)cc21 | IC50: 40.0 nM | 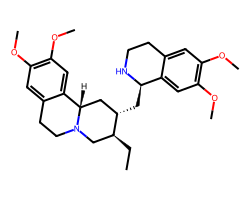 |
| ESMOLOL | COC(=O)CCc1ccc(OCC(O)CNC(C)C)cc1 | Kd: 100.0 nM | 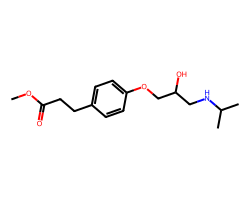 |
| FLUCONAZOLE | OC(Cn1cncn1)(Cn1cncn1)c1ccc(F)cc1F | IC80: 2.8 ug ml-1 | 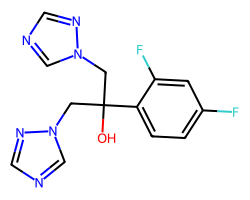 |
| CANDESARTAN | CCOc1nc2cccc(C(=O)O)c2n1Cc1ccc(-c2ccccc2-c2nnn[nH]2)cc1 | T1/2: 9.0 hr | 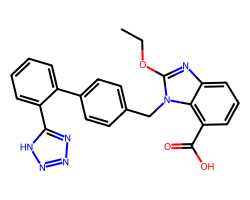 |
| TADALAFIL | CN1CC(=O)N2[C@H](c3ccc4c(c3)OCO4)c3[nH]c4ccccc4c3C[C@@H]2C1=O | IC50: 6.7 nM | 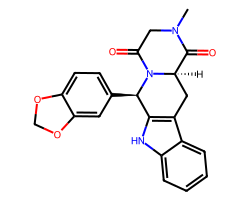 |
| DICHLORPHENAMIDE | NS(=O)(=O)c1cc(Cl)c(Cl)c(S(N)(=O)=O)c1 | Ki: 1200.0 nM | 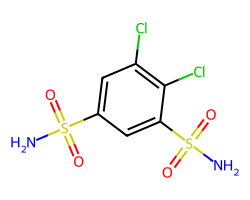 |
| ACEBUTOLOL | CCCC(=O)Nc1ccc(OCC(O)CNC(C)C)c(C(C)=O)c1 | LD50: 76.0 mg.kg-1 | 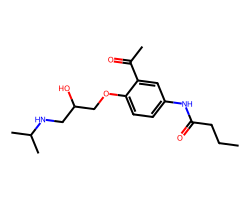 |
| ASTEMIZOLE | COc1ccc(CCN2CCC(Nc3nc4ccccc4n3Cc3ccc(F)cc3)CC2)cc1 | Binding energy: 11.3 kCal mol-1 |  |
| NAFAMOSTAT | N=C(N)Nc1ccc(C(=O)Oc2ccc3cc(C(=N)N)ccc3c2)cc1 | IC50: 12000.0 nM |  |
| QUERCETIN | O=c1c(O)c(-c2ccc(O)c(O)c2)oc2cc(O)cc(O)c12 | IC50: 32870.0 nM |  |
| AMILORIDE | N=C(N)NC(=O)c1nc(Cl)c(N)nc1N | Pc: 780000.0 cm s-1 |  |
| SILDENAFIL | CCCc1nn(C)c2c(=O)[nH]c(-c3cc(S(=O)(=O)N4CCN(C)CC4)ccc3OCC)nc12 | Pc: 87000000.0 cm s-1 | 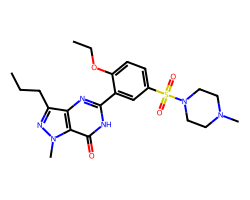 |
| VOGLIBOSE | OCC(CO)N[C@H]1C[C@](O)(CO)[C@@H](O)[C@H](O)[C@H]1O | IC50: 110.0 nM | 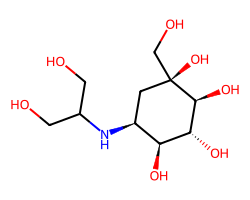 |
| FULVESTRANT | C[C@]12CC[C@@H]3c4ccc(O)cc4C[C@@H](CCCCCCCCC[S+]([O-])CCCC(F)(F)C(F)(F)F)[C@H]3[C@@H]1CC[C@@H]2O | IC50: 0.49 nM | 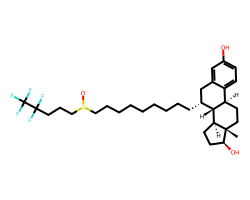 |
| TOFACITINIB | C[C@@H]1CCN(C(=O)CC#N)C[C@@H]1N(C)c1ncnc2[nH]ccc12 | IC50: 13.0 nM | 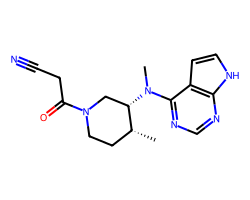 |
| ESTRADIOL | C[C@]12CC[C@@H]3c4ccc(O)cc4CC[C@H]3[C@@H]1CC[C@@H]2O | IC50: 1.5 nM |  |
| OXATOMIDE | O=c1[nH]c2ccccc2n1CCCN1CCN(C(c2ccccc2)c2ccccc2)CC1 | A10: 0.014 mg l-1 |  |
| BAZEDOXIFENE | Cc1c(-c2ccc(O)cc2)n(Cc2ccc(OCCN3CCCCCC3)cc2)c2ccc(O)cc12 | IC50: 23.0 nM |  |
| PENTOSTATIN | OC[C@H]1O[C@@H](n2cnc3c2N=CNC[C@H]3O)C[C@@H]1O | Ki: 0.0025 nM |  |
Top Fragments
| SMILES | Avg pKi | Count | Structure |
|---|---|---|---|
| [3*]O[3*] | 6.79 | 272 | 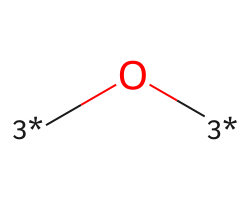 |
| [5*]N[5*] | 7.01 | 225 | 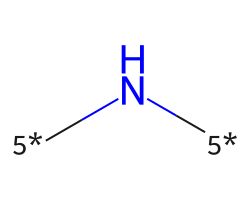 |
| [16*]c1ccc([16*])cc1 | 6.88 | 152 | 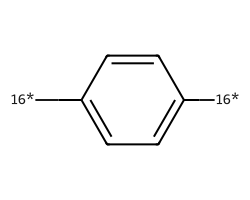 |
| [1*]C([6*])=O | 6.59 | 115 | 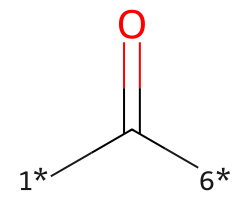 |
| [3*]OC | 7.32 | 113 | 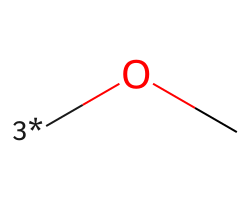 |