Phosphodiesterase 4
Target ID: CHEMBL2093863
Organism: Homo sapiens
Type: PROTEIN FAMILY
Molecules
| Name | SMILES | Bioactivity | Structure |
|---|---|---|---|
| INAMRINONE | Nc1cc(-c2ccncc2)c[nH]c1=O | Mean ED50: 0.389 mg kg-1 | 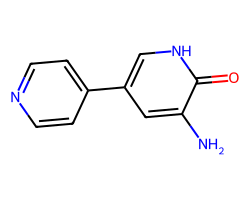 |
| CIPAMFYLLINE | Nc1nc2c([nH]1)c(=O)n(CC1CC1)c(=O)n2CC1CC1 | Inhibition: 5.0 % | 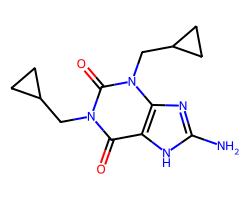 |
| ZAPRINAST | CCCOc1ccccc1-c1nc2[nH]nnc2c(=O)[nH]1 | IC50: 1750000.0 nM | 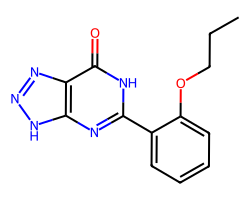 |
| THEOPHYLLINE | Cn1c(=O)c2[nH]cnc2n(C)c1=O | ED50: 18.5 mg.kg-1 | 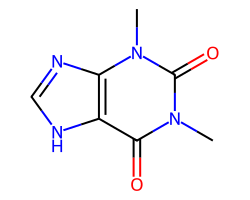 |
| TADALAFIL | CN1CC(=O)N2[C@H](c3ccc4c(c3)OCO4)c3[nH]c4ccccc4c3C[C@@H]2C1=O | IC50: 6.7 nM | 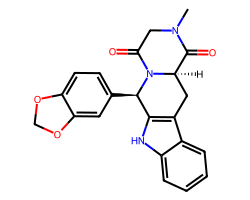 |
| SILDENAFIL | CCCc1nn(C)c2c(=O)[nH]c(-c3cc(S(=O)(=O)N4CCN(C)CC4)ccc3OCC)nc12 | Pc: 87000000.0 cm s-1 | 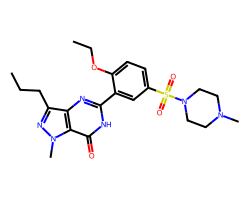 |
| ISOMAZOLE | COc1cc([S+](C)[O-])ccc1-c1nc2cnccc2[nH]1 | ED50: 0.05 dp/dt | 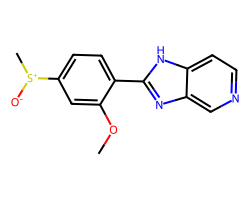 |
| CILOMILAST | COc1ccc([C@]2(C#N)CC[C@@H](C(=O)O)CC2)cc1OC1CCCC1 | IC50: 100.0 nM | 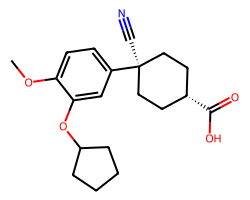 |
| ROLIPRAM | COc1ccc(C2CNC(=O)C2)cc1OC1CCCC1 | IC50: 1000.0 nM | 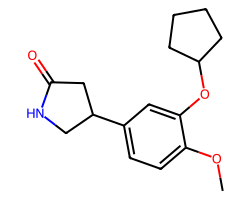 |
| SULMAZOLE | COc1cc([S+](C)[O-])ccc1-c1nc2cccnc2[nH]1 | Delta: 163.0 % | 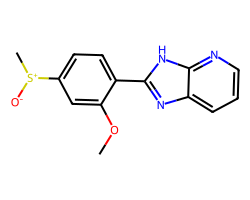 |
| DIPYRIDAMOLE | OCCN(CCO)c1nc(N2CCCCC2)c2nc(N(CCO)CCO)nc(N3CCCCC3)c2n1 | IC50: 500.0 nM | 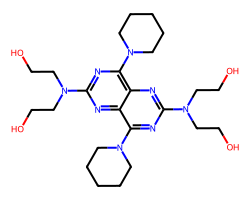 |
Top Fragments
| SMILES | Avg pKi | Count | Structure |
|---|---|---|---|
| [3*]O[3*] | 5.25 | 156 | 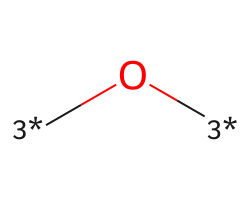 |
| [3*]OC | 6.5 | 156 | 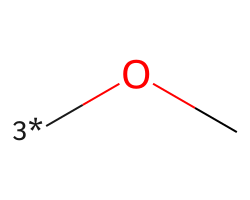 |
| [16*]c1ccc([S+](C)[O-])cc1[16*] | None | 78 | 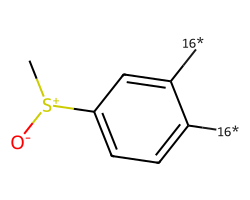 |
| [16*]c1ccc([16*])c([16*])c1 | 6.5 | 78 | 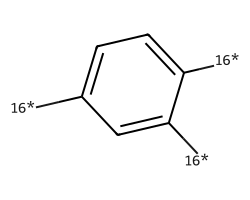 |
| [15*]C1CCCC1 | 6.5 | 78 | 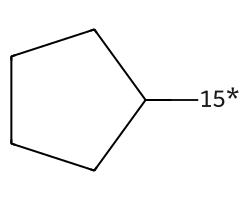 |