Maltase-glucoamylase
Target ID: CHEMBL2074
Organism: Homo sapiens
Type: SINGLE PROTEIN
Molecules
| Name | SMILES | Bioactivity | Structure |
|---|---|---|---|
| GENISTEIN | O=c1c(-c2ccc(O)cc2)coc2cc(O)cc(O)c12 | IC50: 1000.0 nM | 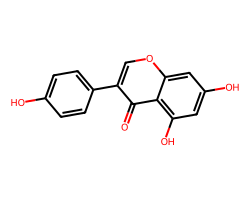 |
| NIZUBAGLUSTAT | OC[C@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1CCCCCOCc1ccc(-c2ccccc2)c(F)c1 | IC50: 2.5 nM | 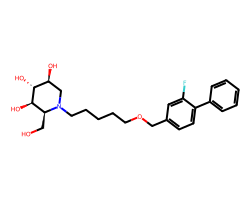 |
| LUCERASTAT | CCCCN1C[C@H](O)[C@@H](O)[C@@H](O)[C@H]1CO | IC50: 10000.0 nM | 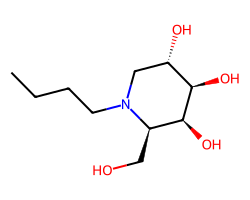 |
| BAICALEIN | O=c1cc(-c2ccccc2)oc2cc(O)c(O)c(O)c12 | Inhibition: 57.0 % | 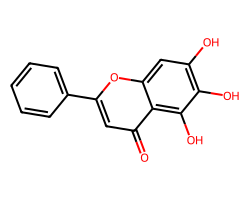 |
| QUERCETIN | O=c1c(O)c(-c2ccc(O)c(O)c2)oc2cc(O)cc(O)c12 | IC50: 32870.0 nM | 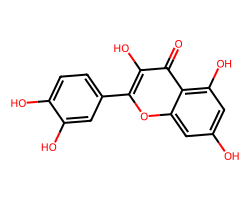 |
| MIGLITOL | OCCN1C[C@H](O)[C@@H](O)[C@H](O)[C@H]1CO | Inhibition: 50.0 % | 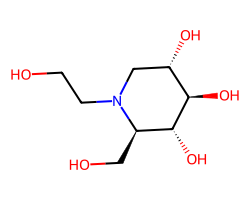 |
| VOGLIBOSE | OCC(CO)N[C@H]1C[C@](O)(CO)[C@@H](O)[C@H](O)[C@H]1O | IC50: 110.0 nM | 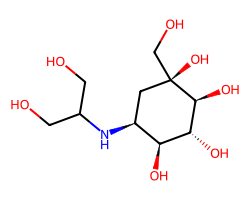 |
| INOSITOL | O[C@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](O)[C@H]1O | k cat: 13.3 s-1 | 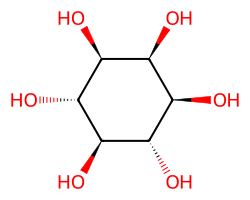 |
| MIGALASTAT | OC[C@H]1NC[C@H](O)[C@@H](O)[C@H]1O | Ki: 34000000.0 nM | 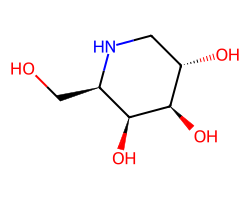 |
| DUVOGLUSTAT | OC[C@H]1NC[C@H](O)[C@@H](O)[C@@H]1O | IC50: 91900.0 nM | 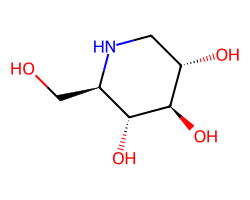 |
| ANDROGRAPHOLIDE | C=C1CC[C@@H]2[C@](C)(CO)[C@H](O)CC[C@@]2(C)[C@@H]1C/C=C1/C(=O)OC[C@H]1O | IG50: 30.0 uM | 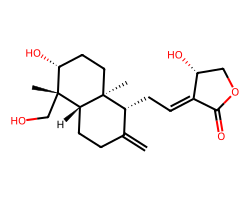 |
| MIGLUSTAT | CCCCN1C[C@H](O)[C@@H](O)[C@H](O)[C@H]1CO | IC50: 2100.0 nM | 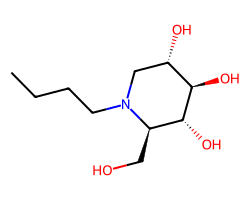 |
Top Fragments
| SMILES | Avg pKi | Count | Structure |
|---|---|---|---|
| [8*]CO | 5.29 | 312 | 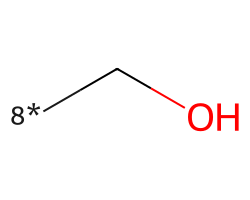 |
| C1CCNCC1 | 4.05 | 195 | 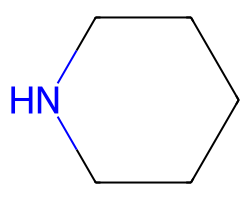 |
| [16*]c1ccccc1 | 8.6 | 78 | 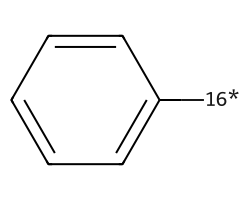 |
| [4*]CCCC | 5.34 | 78 | 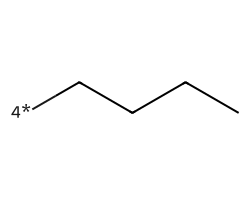 |
| O=c1cc(-c2ccccc2)oc2ccccc12 | 4.48 | 78 |  |