Monoamine oxidase A
Target ID: CHEMBL1951
Organism: Homo sapiens
Type: SINGLE PROTEIN
Molecules
| Name | SMILES | Bioactivity | Structure |
|---|---|---|---|
| AMPHOTERICIN B | C[C@@H]1[C@H](O)[C@@H](C)/C=C/C=C/C=C/C=C/C=C/C=C/C=C/[C@H](O[C@@H]2O[C@H](C)[C@@H](O)[C@H](N)[C@@H]2O)C[C@@H]2O[C@](O)(C[C@@H](O)C[C@@H](O)[C@H](O)CC[C@@H](O)C[C@@H](O)CC(=O)O[C@H]1C)C[C@H](O)[C@H]2C(=O)O | MIC: 4000.0 ug.mL-1 |  |
| ACETAZOLAMIDE | CC(=O)Nc1nnc(S(N)(=O)=O)s1 | Ki: 250.0 nM |  |
| AMPICILLIN | CC1(C)S[C@@H]2[C@H](NC(=O)[C@H](N)c3ccccc3)C(=O)N2[C@H]1C(=O)O | MIC: 5.0 ug.mL-1 |  |
| DICHLORPHENAMIDE | NS(=O)(=O)c1cc(Cl)c(Cl)c(S(N)(=O)=O)c1 | Ki: 1200.0 nM |  |
| QUERCETIN | O=c1c(O)c(-c2ccc(O)c(O)c2)oc2cc(O)cc(O)c12 | IC50: 32870.0 nM | 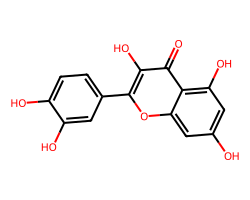 |
| TRIMETHOPRIM | COc1cc(Cc2cnc(N)nc2N)cc(OC)c1OC | IC50: 12000.0 nM | 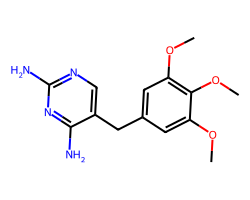 |
Top Fragments
| SMILES | Avg pKi | Count | Structure |
|---|---|---|---|
| [5*]N[5*] | 6.6 | 45 | 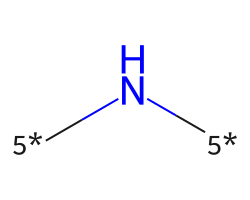 |
| O=c1cc(-c2ccccc2)oc2ccccc12 | 4.48 | 39 | 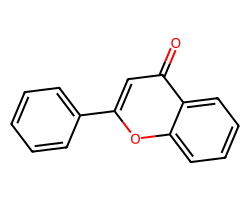 |
| [14*]c1oc2cc(O)cc(O)c2c(=O)c1O | 4.48 | 39 | 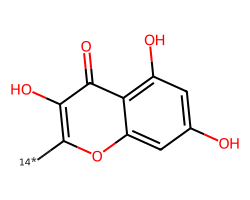 |
| [16*]c1ccc(O)c(O)c1 | 4.48 | 39 | 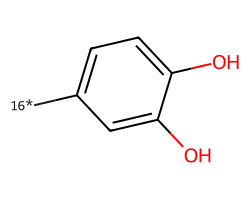 |
| [3*]OC | 4.92 | 39 | 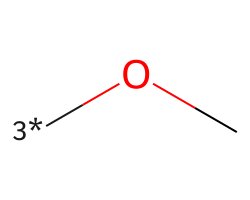 |