Cysteinyl leukotriene receptor 1
Target ID: CHEMBL1798
Organism: Homo sapiens
Type: SINGLE PROTEIN
Molecules
| Name | SMILES | Bioactivity | Structure |
|---|---|---|---|
| ABAMECTIN | CC[C@H](C)[C@H]1O[C@]2(C=C[C@@H]1C)C[C@@H]1C[C@@H](C/C=C(\C)[C@@H](O[C@H]3C[C@H](OC)[C@@H](O[C@H]4C[C@H](OC)[C@@H](O)[C@H](C)O4)[C@H](C)O3)[C@@H](C)/C=C/C=C3\CO[C@@H]4[C@H](O)C(C)=C[C@@H](C(=O)O1)[C@]34O)O2.CO[C@H]1C[C@H](O[C@H]2[C@H](C)O[C@@H](O[C@@H]3/C(C)=C/C[C@@H]4C[C@@H](C[C@]5(C=C[C@H](C)[C@@H](C(C)C)O5)O4)OC(=O)[C@@H]4C=C(C)[C@@H](O)[C@H]5OC/C(=C\C=C\[C@@H]3C)[C@]54O)C[C@@H]2OC)O[C@@H](C)[C@@H]1O | EC50: 12000.0 nM | 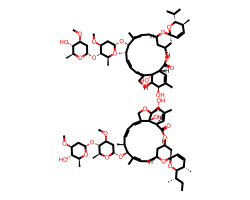 |
| GENISTEIN | O=c1c(-c2ccc(O)cc2)coc2cc(O)cc(O)c12 | IC50: 1000.0 nM | 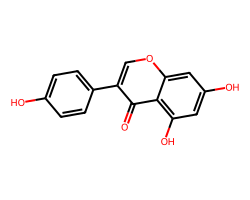 |
| FLUOCINOLONE ACETONIDE | CC1(C)O[C@@H]2C[C@H]3[C@@H]4C[C@H](F)C5=CC(=O)C=C[C@]5(C)[C@@]4(F)[C@@H](O)C[C@]3(C)[C@]2(C(=O)CO)O1 | Potency: 35481.3 nM | 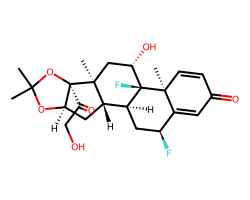 |
| FUROSEMIDE | NS(=O)(=O)c1cc(C(=O)O)c(NCc2ccco2)cc1Cl | Ki: 100000.0 nM | 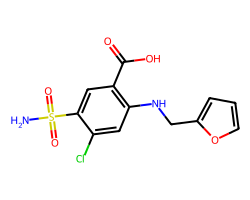 |
| FLUVASTATIN | CC(C)n1c(/C=C/C(O)CC(O)CC(=O)O)c(-c2ccc(F)cc2)c2ccccc21 | IC50: 28.0 nM |  |
| FURAZOLIDONE | O=C1OCCN1/N=C/c1ccc([N+](=O)[O-])o1 | MIC: 77400.0 nM |  |
| HEXACHLOROPHENE | Oc1c(Cl)cc(Cl)c(Cl)c1Cc1c(O)c(Cl)cc(Cl)c1Cl | Control current: 237.4 % |  |
| INAMRINONE | Nc1cc(-c2ccncc2)c[nH]c1=O | Mean ED50: 0.389 mg kg-1 |  |
| HYDROXYPROGESTERONE | CC(=O)[C@@]1(O)CC[C@H]2[C@@H]3CCC4=CC(=O)CC[C@]4(C)[C@H]3CC[C@@]21C | Remaining activity: 89.0 % |  |
| AMINOPHYLLINE | Cn1c(=O)c2[nH]cnc2n(C)c1=O.Cn1c(=O)c2[nH]cnc2n(C)c1=O.NCCN | IC50: 16700.0 nM |  |
| GENTIAN VIOLET | CN(C)c1ccc(C(=C2C=CC(=[N+](C)C)C=C2)c2ccc(N(C)C)cc2)cc1.[Cl-] | Lysis: 100.0 % |  |
| GUAIFENESIN | COc1ccccc1OCC(O)CO | Inhibition: 5.0 % |  |
| 6-METHOXY-2-NAPHTHYLACETIC ACID | COc1ccc2cc(CC(=O)O)ccc2c1 | Inhibition: 5.0 % |  |
| ERGOCALCIFEROL | C=C1CC[C@H](O)C/C1=C/C=C1\CCC[C@]2(C)[C@@H]([C@H](C)/C=C/[C@H](C)C(C)C)CC[C@@H]12 | EC50: 3800.0 nM |  |
| EDARAVONE | CC1=NN(c2ccccc2)C(=O)C1 | PC50: 70.4 uM |  |
| ETHACRYNIC ACID | C=C(CC)C(=O)c1ccc(OCC(=O)O)c(Cl)c1Cl | mequiv of Na+/kg: 4.14 0-5h |  |
| BUFLOMEDIL | COc1cc(OC)c(C(=O)CCCN2CCCC2)c(OC)c1 | Ki: 60.0 nM |  |
| AMITRAZ | Cc1ccc(/N=C/N(C)/C=N/c2ccc(C)cc2C)c(C)c1 | Potency: 0.9 nM |  |
| AMPRENAVIR | CC(C)CN(C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)O[C@H]1CCOC1)S(=O)(=O)c1ccc(N)cc1 | Ki: 0.16 nM |  |
| AMITRIPTYLINE | CN(C)CCC=C1c2ccccc2CCc2ccccc21 | IC50: 61.0 nM |  |
| AMPHOTERICIN B | C[C@@H]1[C@H](O)[C@@H](C)/C=C/C=C/C=C/C=C/C=C/C=C/C=C/[C@H](O[C@@H]2O[C@H](C)[C@@H](O)[C@H](N)[C@@H]2O)C[C@@H]2O[C@](O)(C[C@@H](O)C[C@@H](O)[C@H](O)CC[C@@H](O)C[C@@H](O)CC(=O)O[C@H]1C)C[C@H](O)[C@H]2C(=O)O | MIC: 4000.0 ug.mL-1 | 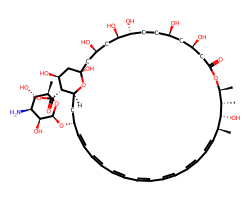 |
| GATIFLOXACIN | COc1c(N2CCNC(C)C2)c(F)cc2c(=O)c(C(=O)O)cn(C3CC3)c12 | MIC: 0.05 ug.mL-1 | 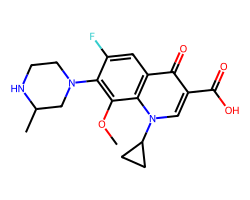 |
| ACETAZOLAMIDE | CC(=O)Nc1nnc(S(N)(=O)=O)s1 | Ki: 250.0 nM | 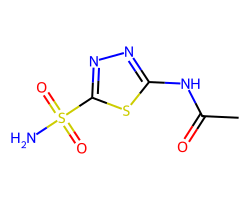 |
| CANTHARIDIN | C[C@@]12C(=O)OC(=O)[C@]1(C)[C@H]1CC[C@@H]2O1 | IC50: 160.0 nM | 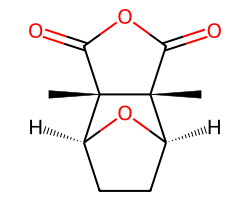 |
| ATENOLOL | CC(C)NCC(O)COc1ccc(CC(N)=O)cc1 | Pc: 1000000.0 cm s-1 | 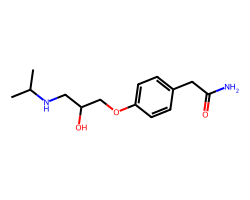 |
| CARBIMAZOLE | CCOC(=O)n1ccn(C)c1=S | IC50: 10400.0 nM | 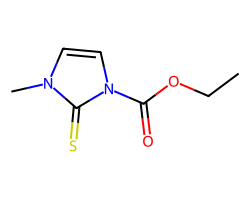 |
| EMETINE | CC[C@H]1CN2CCc3cc(OC)c(OC)cc3[C@@H]2C[C@@H]1C[C@H]1NCCc2cc(OC)c(OC)cc21 | IC50: 40.0 nM | 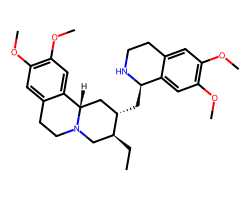 |
| ACLARUBICIN | CC[C@@]1(O)C[C@H](O[C@H]2C[C@H](N(C)C)[C@H](O[C@H]3C[C@H](O)[C@H](O[C@H]4CCC(=O)[C@H](C)O4)[C@H](C)O3)[C@H](C)O2)c2c(cc3c(c2O)C(=O)c2c(O)cccc2C3=O)[C@H]1C(=O)OC | IC50: 9900.0 nM | 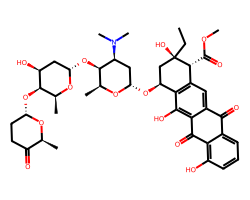 |
| ANISINDIONE | COc1ccc(C2C(=O)c3ccccc3C2=O)cc1 | Inhibition: 1.3 % |  |
| AMINOCAPROIC ACID | NCCCCCC(=O)O | Ki: 50000.0 nM |  |
| BETADEX | OC[C@H]1O[C@@H]2O[C@H]3[C@H](O)[C@@H](O)[C@@H](O[C@H]4[C@H](O)[C@@H](O)[C@@H](O[C@H]5[C@H](O)[C@@H](O)[C@@H](O[C@H]6[C@H](O)[C@@H](O)[C@@H](O[C@H]7[C@H](O)[C@@H](O)[C@@H](O[C@H]8[C@H](O)[C@@H](O)[C@@H](O[C@H]1[C@H](O)[C@H]2O)O[C@@H]8CO)O[C@@H]7CO)O[C@@H]6CO)O[C@@H]5CO)O[C@@H]4CO)O[C@@H]3CO | k_obs: 4.16e-07 s-1 |  |
| BRIMONIDINE | Brc1c(NC2=NCCN2)ccc2nccnc12 | pC25: 1.55 uM kg-1 |  |
| ESMOLOL | COC(=O)CCc1ccc(OCC(O)CNC(C)C)cc1 | Kd: 100.0 nM |  |
| FLUCONAZOLE | OC(Cn1cncn1)(Cn1cncn1)c1ccc(F)cc1F | IC80: 2.8 ug ml-1 |  |
| AMIKACIN | NCC[C@H](O)C(=O)N[C@@H]1C[C@H](N)[C@@H](O[C@H]2O[C@H](CN)[C@@H](O)[C@H](O)[C@H]2O)[C@H](O)[C@H]1O[C@H]1O[C@H](CO)[C@@H](O)[C@H](N)[C@H]1O | C50: 0.21 uM |  |
| CANDESARTAN | CCOc1nc2cccc(C(=O)O)c2n1Cc1ccc(-c2ccccc2-c2nnn[nH]2)cc1 | T1/2: 9.0 hr |  |
| DIETHYLSTILBESTROL | CC/C(=C(/CC)c1ccc(O)cc1)c1ccc(O)cc1 | EC50: 9.0 nM |  |
| FERULATE | COc1cc(/C=C/C(=O)O)ccc1O | Inhibition: 12.0 % |  |
| ACETAMINOPHEN | CC(=O)Nc1ccc(O)cc1 | Vdss: 0.95 L.kg-1 |  |
| EUCALYPTOL | CC12CCC(CC1)C(C)(C)O2 | MIC: 800.0 ug.mL-1 |  |
| HALOPROGIN | Clc1cc(Cl)c(OCC#CI)cc1Cl | MIC: 3.13 ug.mL-1 |  |
| AMPICILLIN | CC1(C)S[C@@H]2[C@H](NC(=O)[C@H](N)c3ccccc3)C(=O)N2[C@H]1C(=O)O | MIC: 5.0 ug.mL-1 |  |
| AZARIBINE | CC(=O)OC[C@H]1O[C@@H](n2ncc(=O)[nH]c2=O)[C@H](OC(C)=O)[C@@H]1OC(C)=O | Potency: 562.3 nM |  |
| ACEBUTOLOL | CCCC(=O)Nc1ccc(OCC(O)CNC(C)C)c(C(C)=O)c1 | LD50: 76.0 mg.kg-1 |  |
| FLUNARIZINE | Fc1ccc(C(c2ccc(F)cc2)N2CCN(C/C=C/c3ccccc3)CC2)cc1 | IC50: 290.0 nM |  |
| ASTROMICIN | CO[C@H]1[C@@H](O)[C@H](N)[C@@H](O[C@H]2O[C@H]([C@H](C)N)CC[C@H]2N)[C@H](O)[C@@H]1N(C)C(=O)CN | Hepatotoxicity (moderate): 7.3 % |  |
| BITHIONOL | Oc1c(Cl)cc(Cl)cc1Sc1cc(Cl)cc(Cl)c1O | Control current: 198.6 % |  |
| FLOXURIDINE | O=c1[nH]c(=O)n([C@H]2C[C@H](O)[C@@H](CO)O2)cc1F | IC50: 2.0 nM |  |
| ETHYNODIOL DIACETATE | C#C[C@]1(OC(C)=O)CC[C@H]2[C@@H]3CCC4=C[C@@H](OC(C)=O)CC[C@@H]4[C@H]3CC[C@@]21C | Potency: 15848.9 nM |  |
| BETAMETHASONE PHOSPHORIC ACID | C[C@H]1C[C@H]2[C@@H]3CCC4=CC(=O)C=C[C@]4(C)[C@@]3(F)[C@@H](O)C[C@]2(C)[C@@]1(O)C(=O)COP(=O)(O)O | Inhibition: 96.0 % |  |
| AMOXICILLIN | CC1(C)S[C@@H]2[C@H](NC(=O)[C@H](N)c3ccc(O)cc3)C(=O)N2[C@H]1C(=O)O | MIC: 0.021 ug.mL-1 |  |
| GLYCYRRHIZIN | CC1(C)[C@@H](O[C@H]2O[C@H](C(=O)O)[C@@H](O)[C@H](O)[C@H]2O[C@@H]2O[C@H](C(=O)O)[C@@H](O)[C@H](O)[C@H]2O)CC[C@]2(C)[C@H]3C(=O)C=C4[C@@H]5C[C@@](C)(C(=O)O)CC[C@]5(C)CC[C@@]4(C)[C@]3(C)CC[C@@H]12 | Protection: 15.0 % |  |
| ACONITINE | CCN1C[C@]2(COC)[C@H](O)C[C@H](OC)[C@@]34[C@@H]5C[C@@]6(O)[C@H](OC(=O)c7ccccc7)[C@@H]5[C@@](OC(C)=O)([C@@H]([C@H](OC)[C@H]23)[C@@H]14)[C@@H](O)[C@@H]6OC | Activity: 160.0 bpm |  |
| AMIFOSTINE | NCCCNCCSP(=O)(O)O | LD50: 1049.0 mg.kg-1 | 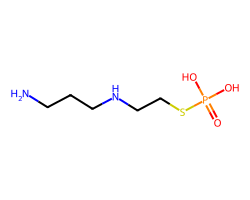 |
| ASTEMIZOLE | COc1ccc(CCN2CCC(Nc3nc4ccccc4n3Cc3ccc(F)cc3)CC2)cc1 | Binding energy: 11.3 kCal mol-1 | 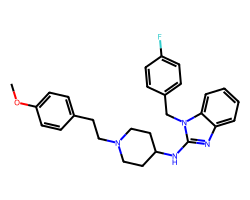 |
| ATROPINE | CN1[C@@H]2CC[C@H]1C[C@@H](OC(=O)C(CO)c1ccccc1)C2 | Ki: 0.45 nM | 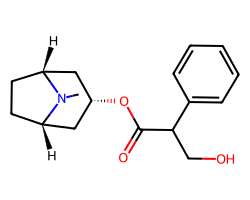 |
| CAFFEINE | Cn1c(=O)c2c(ncn2C)n(C)c1=O | Vdss: 0.61 L.kg-1 | 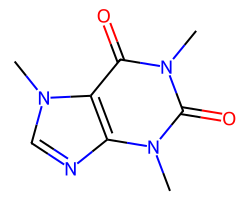 |
| AMILORIDE | N=C(N)NC(=O)c1nc(Cl)c(N)nc1N | Pc: 780000.0 cm s-1 |  |
| HYDROCHLOROTHIAZIDE | NS(=O)(=O)c1cc2c(cc1Cl)NCNS2(=O)=O | IC50: 181970085860998.25 nM |  |
| AMINOPTERIN | Nc1nc(N)c2nc(CNc3ccc(C(=O)N[C@@H](CCC(=O)O)C(=O)O)cc3)cnc2n1 | Ki: 0.0037 nM |  |
| ESTRADIOL | C[C@]12CC[C@@H]3c4ccc(O)cc4CC[C@H]3[C@@H]1CC[C@@H]2O | IC50: 1.5 nM |  |
| CILOSTAMIDE | CN(C(=O)CCCOc1ccc2[nH]c(=O)ccc2c1)C1CCCCC1 | EC50: 1200.0 nM |  |
| IMATINIB | Cc1ccc(NC(=O)c2ccc(CN3CCN(C)CC3)cc2)cc1Nc1nccc(-c2cccnc2)n1 | IC50: 40.0 nM |  |
| ESTRONE | C[C@]12CC[C@@H]3c4ccc(O)cc4CC[C@H]3[C@@H]1CCC2=O | EC50: 1135.0 nM |  |
| DIPYRIDAMOLE | OCCN(CCO)c1nc(N2CCCCC2)c2nc(N(CCO)CCO)nc(N3CCCCC3)c2n1 | IC50: 500.0 nM |  |
Top Fragments
| SMILES | Avg pKi | Count | Structure |
|---|---|---|---|
| [3*]O[3*] | 6.22 | 293 | 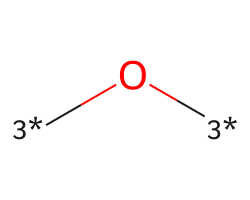 |
| [5*]N[5*] | 7.92 | 249 | 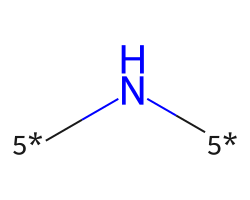 |
| [3*]OC | 6.2 | 197 | 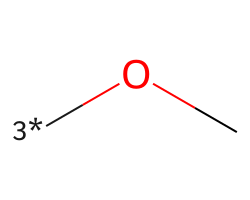 |
| [16*]c1ccc([16*])cc1 | 9.07 | 162 | 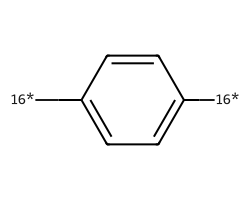 |
| [1*]C([6*])=O | 9.41 | 122 | 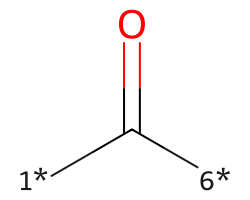 |