Solute carrier organic anion transporter family member 1B3
Target ID: CHEMBL1743121
Organism: Homo sapiens
Type: SINGLE PROTEIN
Molecules
| Name | SMILES | Bioactivity | Structure |
|---|---|---|---|
| METHOTREXATE | CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(C(=O)N[C@@H](CCC(=O)O)C(=O)O)cc1 | ID50: 6.2 nM |  |
| GENISTEIN | O=c1c(-c2ccc(O)cc2)coc2cc(O)cc(O)c12 | IC50: 1000.0 nM |  |
| FUROSEMIDE | NS(=O)(=O)c1cc(C(=O)O)c(NCc2ccco2)cc1Cl | Ki: 100000.0 nM |  |
| FLUVASTATIN | CC(C)n1c(/C=C/C(O)CC(O)CC(=O)O)c(-c2ccc(F)cc2)c2ccccc21 | IC50: 28.0 nM |  |
| FURAZOLIDONE | O=C1OCCN1/N=C/c1ccc([N+](=O)[O-])o1 | MIC: 77400.0 nM | 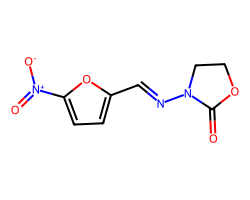 |
| HEXACHLOROPHENE | Oc1c(Cl)cc(Cl)c(Cl)c1Cc1c(O)c(Cl)cc(Cl)c1Cl | Control current: 237.4 % | 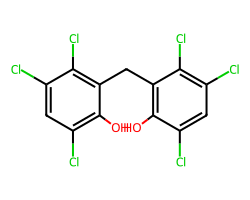 |
| GUAIFENESIN | COc1ccccc1OCC(O)CO | Inhibition: 5.0 % | 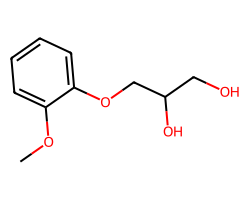 |
| ERGOCALCIFEROL | C=C1CC[C@H](O)C/C1=C/C=C1\CCC[C@]2(C)[C@@H]([C@H](C)/C=C/[C@H](C)C(C)C)CC[C@@H]12 | EC50: 3800.0 nM | 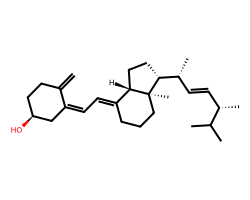 |
| ETHACRYNIC ACID | C=C(CC)C(=O)c1ccc(OCC(=O)O)c(Cl)c1Cl | mequiv of Na+/kg: 4.14 0-5h |  |
| MIFEPRISTONE | CC#C[C@]1(O)CC[C@H]2[C@@H]3CCC4=CC(=O)CCC4=C3[C@@H](c3ccc(N(C)C)cc3)C[C@@]21C | IC50: 0.028 nM |  |
| ERLOTINIB | C#Cc1cccc(Nc2ncnc3cc(OCCOC)c(OCCOC)cc23)c1 | IC50: 1450.0 nM |  |
| ROSUVASTATIN | CC(C)c1nc(N(C)S(C)(=O)=O)nc(-c2ccc(F)cc2)c1/C=C/[C@@H](O)C[C@@H](O)CC(=O)O | Ki: 0.9 nM |  |
| AMPRENAVIR | CC(C)CN(C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)O[C@H]1CCOC1)S(=O)(=O)c1ccc(N)cc1 | Ki: 0.16 nM |  |
| AMITRIPTYLINE | CN(C)CCC=C1c2ccccc2CCc2ccccc21 | IC50: 61.0 nM |  |
| AMPHOTERICIN B | C[C@@H]1[C@H](O)[C@@H](C)/C=C/C=C/C=C/C=C/C=C/C=C/C=C/[C@H](O[C@@H]2O[C@H](C)[C@@H](O)[C@H](N)[C@@H]2O)C[C@@H]2O[C@](O)(C[C@@H](O)C[C@@H](O)[C@H](O)CC[C@@H](O)C[C@@H](O)CC(=O)O[C@H]1C)C[C@H](O)[C@H]2C(=O)O | MIC: 4000.0 ug.mL-1 |  |
| GATIFLOXACIN | COc1c(N2CCNC(C)C2)c(F)cc2c(=O)c(C(=O)O)cn(C3CC3)c12 | MIC: 0.05 ug.mL-1 |  |
| ATENOLOL | CC(C)NCC(O)COc1ccc(CC(N)=O)cc1 | Pc: 1000000.0 cm s-1 | 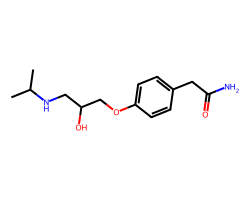 |
| CARBIMAZOLE | CCOC(=O)n1ccn(C)c1=S | IC50: 10400.0 nM | 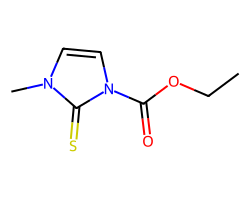 |
| THEOPHYLLINE | Cn1c(=O)c2[nH]cnc2n(C)c1=O | ED50: 18.5 mg.kg-1 | 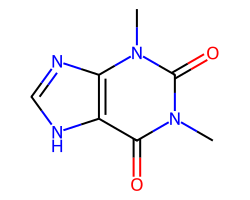 |
| FLUCONAZOLE | OC(Cn1cncn1)(Cn1cncn1)c1ccc(F)cc1F | IC80: 2.8 ug ml-1 | 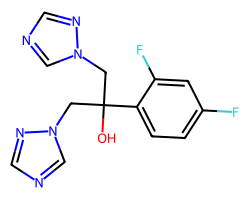 |
| CANDESARTAN | CCOc1nc2cccc(C(=O)O)c2n1Cc1ccc(-c2ccccc2-c2nnn[nH]2)cc1 | T1/2: 9.0 hr |  |
| DIETHYLSTILBESTROL | CC/C(=C(/CC)c1ccc(O)cc1)c1ccc(O)cc1 | EC50: 9.0 nM |  |
| FERULATE | COc1cc(/C=C/C(=O)O)ccc1O | Inhibition: 12.0 % |  |
| BAICALEIN | O=c1cc(-c2ccccc2)oc2cc(O)c(O)c(O)c12 | Inhibition: 57.0 % |  |
| GLYCYRRHIZIN | CC1(C)[C@@H](O[C@H]2O[C@H](C(=O)O)[C@@H](O)[C@H](O)[C@H]2O[C@@H]2O[C@H](C(=O)O)[C@@H](O)[C@H](O)[C@H]2O)CC[C@]2(C)[C@H]3C(=O)C=C4[C@@H]5C[C@@](C)(C(=O)O)CC[C@]5(C)CC[C@@]4(C)[C@]3(C)CC[C@@H]12 | Protection: 15.0 % | 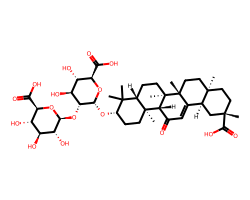 |
| ACONITINE | CCN1C[C@]2(COC)[C@H](O)C[C@H](OC)[C@@]34[C@@H]5C[C@@]6(O)[C@H](OC(=O)c7ccccc7)[C@@H]5[C@@](OC(C)=O)([C@@H]([C@H](OC)[C@H]23)[C@@H]14)[C@@H](O)[C@@H]6OC | Activity: 160.0 bpm | 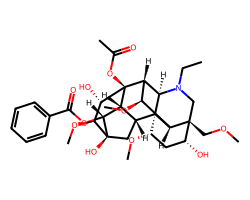 |
| ASTEMIZOLE | COc1ccc(CCN2CCC(Nc3nc4ccccc4n3Cc3ccc(F)cc3)CC2)cc1 | Binding energy: 11.3 kCal mol-1 | 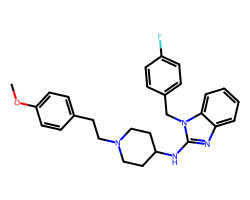 |
| CAFFEINE | Cn1c(=O)c2c(ncn2C)n(C)c1=O | Vdss: 0.61 L.kg-1 | 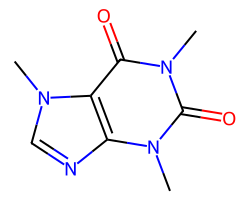 |
| ADENOSINE | Nc1ncnc2c1ncn2[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O | Km: 42000.0 nM |  |
| VALDECOXIB | Cc1onc(-c2ccccc2)c1-c1ccc(S(N)(=O)=O)cc1 | Ki: 54000.0 nM |  |
| QUERCETIN | O=c1c(O)c(-c2ccc(O)c(O)c2)oc2cc(O)cc(O)c12 | IC50: 32870.0 nM |  |
| DAIDZEIN | O=c1c(-c2ccc(O)cc2)coc2cc(O)ccc12 | Suppression: 22.0 % |  |
| MIGLITOL | OCCN1C[C@H](O)[C@@H](O)[C@H](O)[C@H]1CO | Inhibition: 50.0 % | 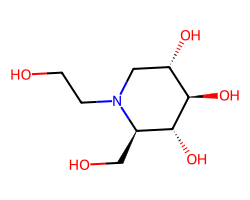 |
| SILDENAFIL | CCCc1nn(C)c2c(=O)[nH]c(-c3cc(S(=O)(=O)N4CCN(C)CC4)ccc3OCC)nc12 | Pc: 87000000.0 cm s-1 | 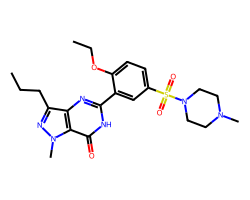 |
| TRIMETHOPRIM | COc1cc(Cc2cnc(N)nc2N)cc(OC)c1OC | IC50: 12000.0 nM | 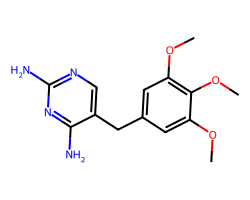 |
| ESTRADIOL | C[C@]12CC[C@@H]3c4ccc(O)cc4CC[C@H]3[C@@H]1CC[C@@H]2O | IC50: 1.5 nM | 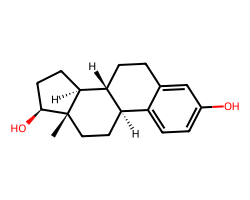 |
| ANDROGRAPHOLIDE | C=C1CC[C@@H]2[C@](C)(CO)[C@H](O)CC[C@@]2(C)[C@@H]1C/C=C1/C(=O)OC[C@H]1O | IG50: 30.0 uM |  |
| TAMOXIFEN | CC/C(=C(\c1ccccc1)c1ccc(OCCN(C)C)cc1)c1ccccc1 | IC50: 6750.0 nM |  |
| SULMAZOLE | COc1cc([S+](C)[O-])ccc1-c1nc2cccnc2[nH]1 | Delta: 163.0 % |  |
| ESTRONE | C[C@]12CC[C@@H]3c4ccc(O)cc4CC[C@H]3[C@@H]1CCC2=O | EC50: 1135.0 nM |  |
| METHAZOLAMIDE | CC(=O)/N=c1/sc(S(N)(=O)=O)nn1C | Ki: 50.0 nM | 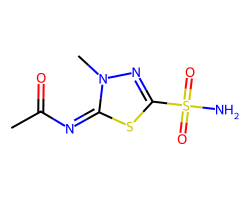 |
| DIPYRIDAMOLE | OCCN(CCO)c1nc(N2CCCCC2)c2nc(N(CCO)CCO)nc(N3CCCCC3)c2n1 | IC50: 500.0 nM | 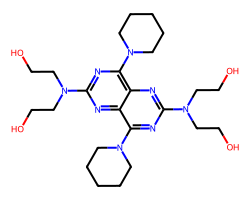 |
Top Fragments
| SMILES | Avg pKi | Count | Structure |
|---|---|---|---|
| [3*]O[3*] | 6.26 | 241 | 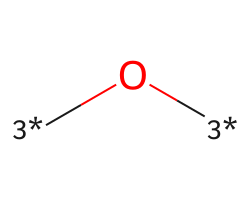 |
| [3*]OC | 5.38 | 180 | 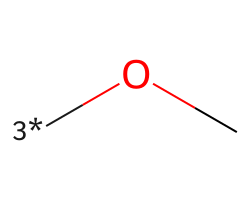 |
| [5*]N[5*] | 6.86 | 143 | 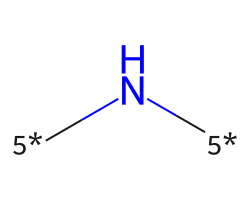 |
| [16*]c1ccc([16*])cc1 | 8.08 | 132 | 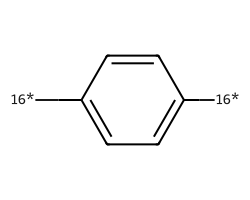 |
| [16*]c1ccccc1 | 5.88 | 126 | 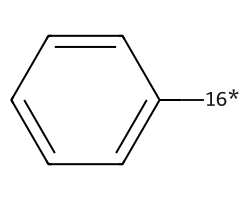 |