Chromobox protein homolog 1
Target ID: CHEMBL1741193
Organism: Homo sapiens
Type: SINGLE PROTEIN
Molecules
| Name | SMILES | Bioactivity | Structure |
|---|---|---|---|
| METHOTREXATE | CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(C(=O)N[C@@H](CCC(=O)O)C(=O)O)cc1 | ID50: 6.2 nM | 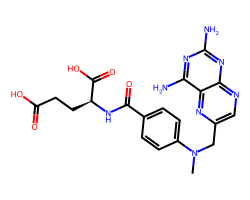 |
| PAPAVERINE | COc1ccc(Cc2nccc3cc(OC)c(OC)cc23)cc1OC | Blood pressure: -23.0 mmHg | 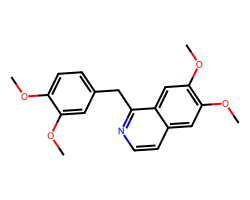 |
| MIFEPRISTONE | CC#C[C@]1(O)CC[C@H]2[C@@H]3CCC4=CC(=O)CCC4=C3[C@@H](c3ccc(N(C)C)cc3)C[C@@]21C | IC50: 0.028 nM | 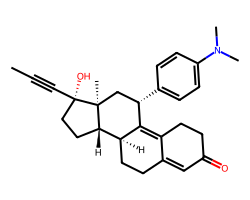 |
| AMITRIPTYLINE | CN(C)CCC=C1c2ccccc2CCc2ccccc21 | IC50: 61.0 nM | 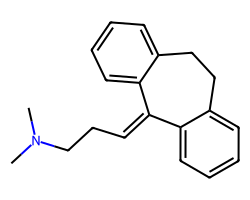 |
| DALFAMPRIDINE | Nc1ccncc1 | Ki: 2000000.0 nM | 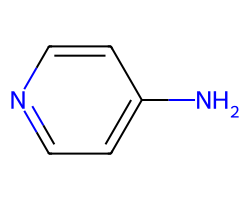 |
| AMINOCAPROIC ACID | NCCCCCC(=O)O | Ki: 50000.0 nM | 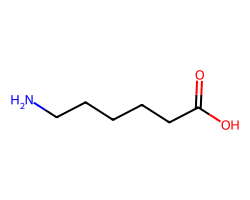 |
| BRIMONIDINE | Brc1c(NC2=NCCN2)ccc2nccnc12 | pC25: 1.55 uM kg-1 | 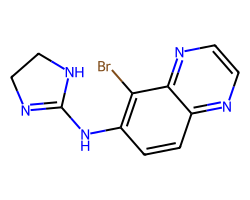 |
| FLUNARIZINE | Fc1ccc(C(c2ccc(F)cc2)N2CCN(C/C=C/c3ccccc3)CC2)cc1 | IC50: 290.0 nM | 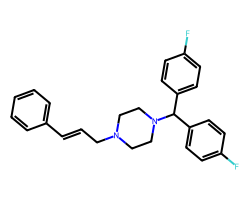 |
| 2-METHOXYESTRADIOL | COc1cc2c(cc1O)CC[C@@H]1[C@@H]2CC[C@]2(C)[C@@H](O)CC[C@@H]12 | GI50: 700.0 nM | 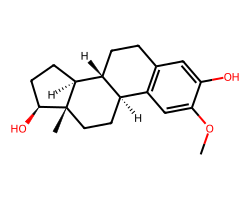 |
| CAFFEINE | Cn1c(=O)c2c(ncn2C)n(C)c1=O | Vdss: 0.61 L.kg-1 | 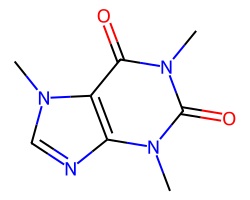 |
| SEMAXANIB | Cc1cc(C)c(/C=C2\C(=O)Nc3ccccc32)[nH]1 | IC50: 700.0 nM | 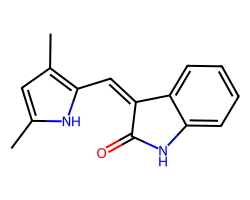 |
| QUERCETIN | O=c1c(O)c(-c2ccc(O)c(O)c2)oc2cc(O)cc(O)c12 | IC50: 32870.0 nM | 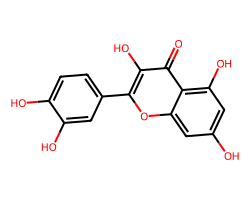 |
| LEVODOPA | N[C@@H](Cc1ccc(O)c(O)c1)C(=O)O | IC50: 900000.0 nM |  |
| HYDROCHLOROTHIAZIDE | NS(=O)(=O)c1cc2c(cc1Cl)NCNS2(=O)=O | IC50: 181970085860998.25 nM |  |
| TRIMETHOPRIM | COc1cc(Cc2cnc(N)nc2N)cc(OC)c1OC | IC50: 12000.0 nM |  |
| NAFTOPIDIL | COc1ccccc1N1CCN(CC(O)COc2cccc3ccccc23)CC1 | Ki: 39.0 nM |  |
| AMINOPTERIN | Nc1nc(N)c2nc(CNc3ccc(C(=O)N[C@@H](CCC(=O)O)C(=O)O)cc3)cnc2n1 | Ki: 0.0037 nM | 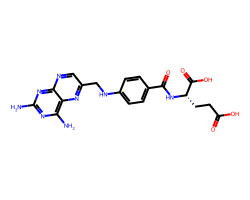 |
| TAMOXIFEN | CC/C(=C(\c1ccccc1)c1ccc(OCCN(C)C)cc1)c1ccccc1 | IC50: 6750.0 nM | 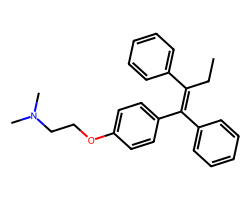 |
| PINACIDIL ANHYDROUS | CC(N/C(=N\C#N)Nc1ccncc1)C(C)(C)C | IC50: 800.0 nM | 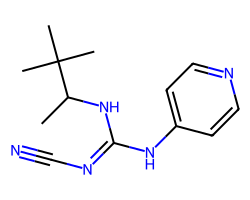 |
| RALOXIFENE | O=C(c1ccc(OCCN2CCCCC2)cc1)c1c(-c2ccc(O)cc2)sc2cc(O)ccc12 | IC50: 1.8 nM | 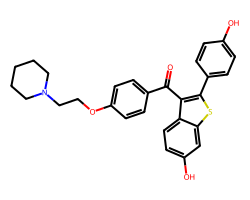 |
| MIBEFRADIL | COCC(=O)O[C@]1(CCN(C)CCCc2nc3ccccc3[nH]2)CCc2cc(F)ccc2[C@@H]1C(C)C | Inhibition: 86.0 % | 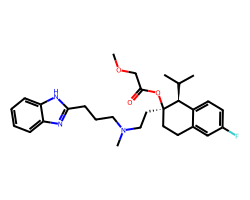 |
Top Fragments
| SMILES | Avg pKi | Count | Structure |
|---|---|---|---|
| [3*]OC | 6.16 | 186 | 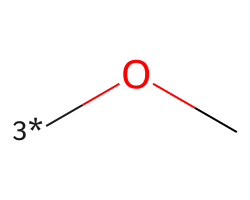 |
| [16*]c1ccc([16*])cc1 | 8.99 | 164 | 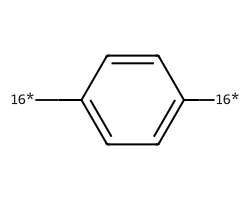 |
| [3*]O[3*] | 7.13 | 133 | 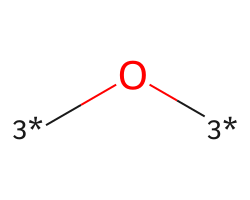 |
| c1ccc(NCc2cnc3ncncc3n2)cc1 | 9.82 | 78 | 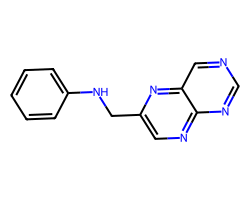 |
| [5*]N[5*] | 9.82 | 78 | 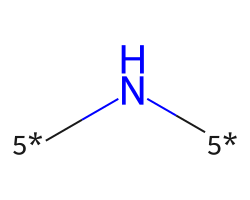 |