Bloom syndrome protein
Target ID: CHEMBL1293237
Organism: Homo sapiens
Type: SINGLE PROTEIN
Molecules
| Name | SMILES | Bioactivity | Structure |
|---|---|---|---|
| METHOTREXATE | CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(C(=O)N[C@@H](CCC(=O)O)C(=O)O)cc1 | ID50: 6.2 nM | 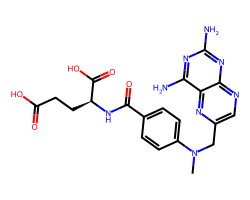 |
| GENISTEIN | O=c1c(-c2ccc(O)cc2)coc2cc(O)cc(O)c12 | IC50: 1000.0 nM | 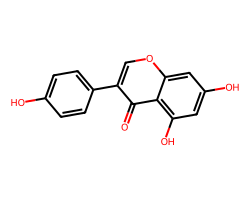 |
| HYDROXYPROGESTERONE | CC(=O)[C@@]1(O)CC[C@H]2[C@@H]3CCC4=CC(=O)CC[C@]4(C)[C@H]3CC[C@@]21C | Remaining activity: 89.0 % | 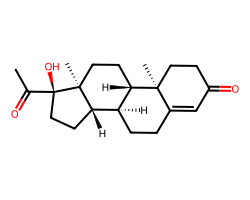 |
| MIFEPRISTONE | CC#C[C@]1(O)CC[C@H]2[C@@H]3CCC4=CC(=O)CCC4=C3[C@@H](c3ccc(N(C)C)cc3)C[C@@]21C | IC50: 0.028 nM | 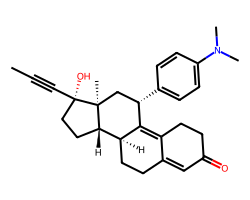 |
| ZAPRINAST | CCCOc1ccccc1-c1nc2[nH]nnc2c(=O)[nH]1 | IC50: 1750000.0 nM |  |
| ACETAZOLAMIDE | CC(=O)Nc1nnc(S(N)(=O)=O)s1 | Ki: 250.0 nM |  |
| BRIMONIDINE | Brc1c(NC2=NCCN2)ccc2nccnc12 | pC25: 1.55 uM kg-1 |  |
| AMIFOSTINE | NCCCNCCSP(=O)(O)O | LD50: 1049.0 mg.kg-1 |  |
| 2-METHOXYESTRADIOL | COc1cc2c(cc1O)CC[C@@H]1[C@@H]2CC[C@]2(C)[C@@H](O)CC[C@@H]12 | GI50: 700.0 nM | 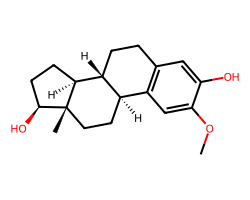 |
| SEMAXANIB | Cc1cc(C)c(/C=C2\C(=O)Nc3ccccc32)[nH]1 | IC50: 700.0 nM | 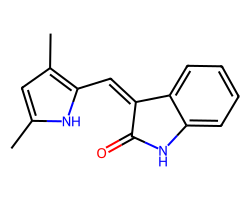 |
| QUERCETIN | O=c1c(O)c(-c2ccc(O)c(O)c2)oc2cc(O)cc(O)c12 | IC50: 32870.0 nM | 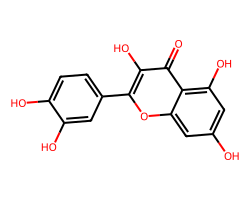 |
| DAIDZEIN | O=c1c(-c2ccc(O)cc2)coc2cc(O)ccc12 | Suppression: 22.0 % | 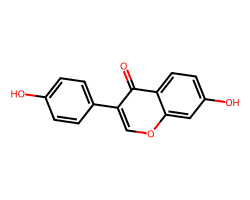 |
| ZARDAVERINE | COc1cc(-c2ccc(=O)[nH]n2)ccc1OC(F)F | IC50: 10000.0 nM | 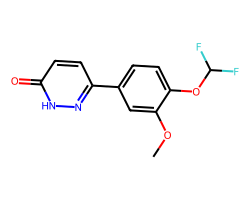 |
| TRIMETHOPRIM | COc1cc(Cc2cnc(N)nc2N)cc(OC)c1OC | IC50: 12000.0 nM | 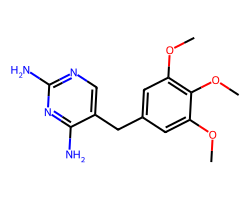 |
| RALOXIFENE HYDROCHLORIDE | Cl.O=C(c1ccc(OCCN2CCCCC2)cc1)c1c(-c2ccc(O)cc2)sc2cc(O)ccc12 | IC50: 0.4 nM | 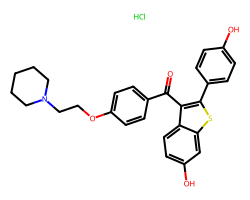 |
| AMINOPTERIN | Nc1nc(N)c2nc(CNc3ccc(C(=O)N[C@@H](CCC(=O)O)C(=O)O)cc3)cnc2n1 | Ki: 0.0037 nM | 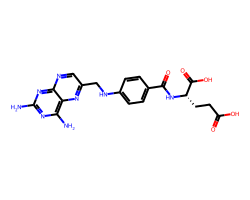 |
| ESTRADIOL | C[C@]12CC[C@@H]3c4ccc(O)cc4CC[C@H]3[C@@H]1CC[C@@H]2O | IC50: 1.5 nM | 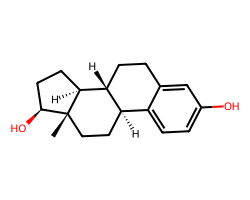 |
| CILOSTAMIDE | CN(C(=O)CCCOc1ccc2[nH]c(=O)ccc2c1)C1CCCCC1 | EC50: 1200.0 nM | 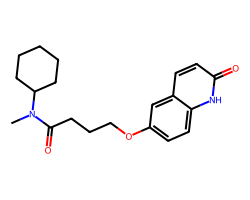 |
| ASPARTIC ACID | N[C@@H](CC(=O)O)C(=O)O | KA: 48.0 uM | 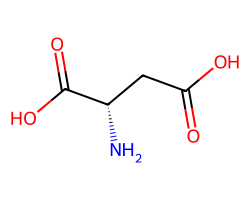 |
| ROLIPRAM | COc1ccc(C2CNC(=O)C2)cc1OC1CCCC1 | IC50: 1000.0 nM | 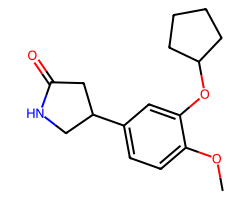 |
| OXATOMIDE | O=c1[nH]c2ccccc2n1CCCN1CCN(C(c2ccccc2)c2ccccc2)CC1 | A10: 0.014 mg l-1 |  |
| ESTRONE | C[C@]12CC[C@@H]3c4ccc(O)cc4CC[C@H]3[C@@H]1CCC2=O | EC50: 1135.0 nM |  |
| PINACIDIL ANHYDROUS | CC(N/C(=N\C#N)Nc1ccncc1)C(C)(C)C | IC50: 800.0 nM |  |
| DIPYRIDAMOLE | OCCN(CCO)c1nc(N2CCCCC2)c2nc(N(CCO)CCO)nc(N3CCCCC3)c2n1 | IC50: 500.0 nM |  |
| MILRINONE | Cc1[nH]c(=O)c(C#N)cc1-c1ccncc1 | Mean ED50: 0.037 mg kg-1 | 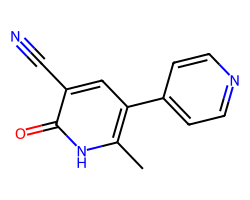 |
Top Fragments
| SMILES | Avg pKi | Count | Structure |
|---|---|---|---|
| [3*]OC | 5.48 | 147 | 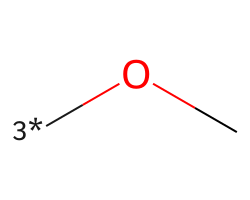 |
| [3*]O[3*] | 5.79 | 146 | 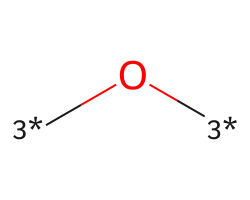 |
| [16*]c1ccc([16*])cc1 | 9.9 | 138 | 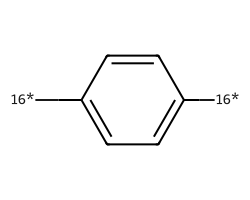 |
| [5*]N[5*] | 8.82 | 119 | 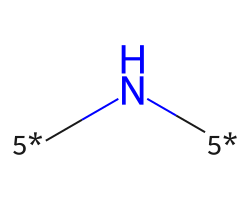 |
| [16*]c1ccc(O)cc1 | 7.45 | 97 | 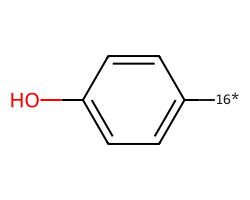 |